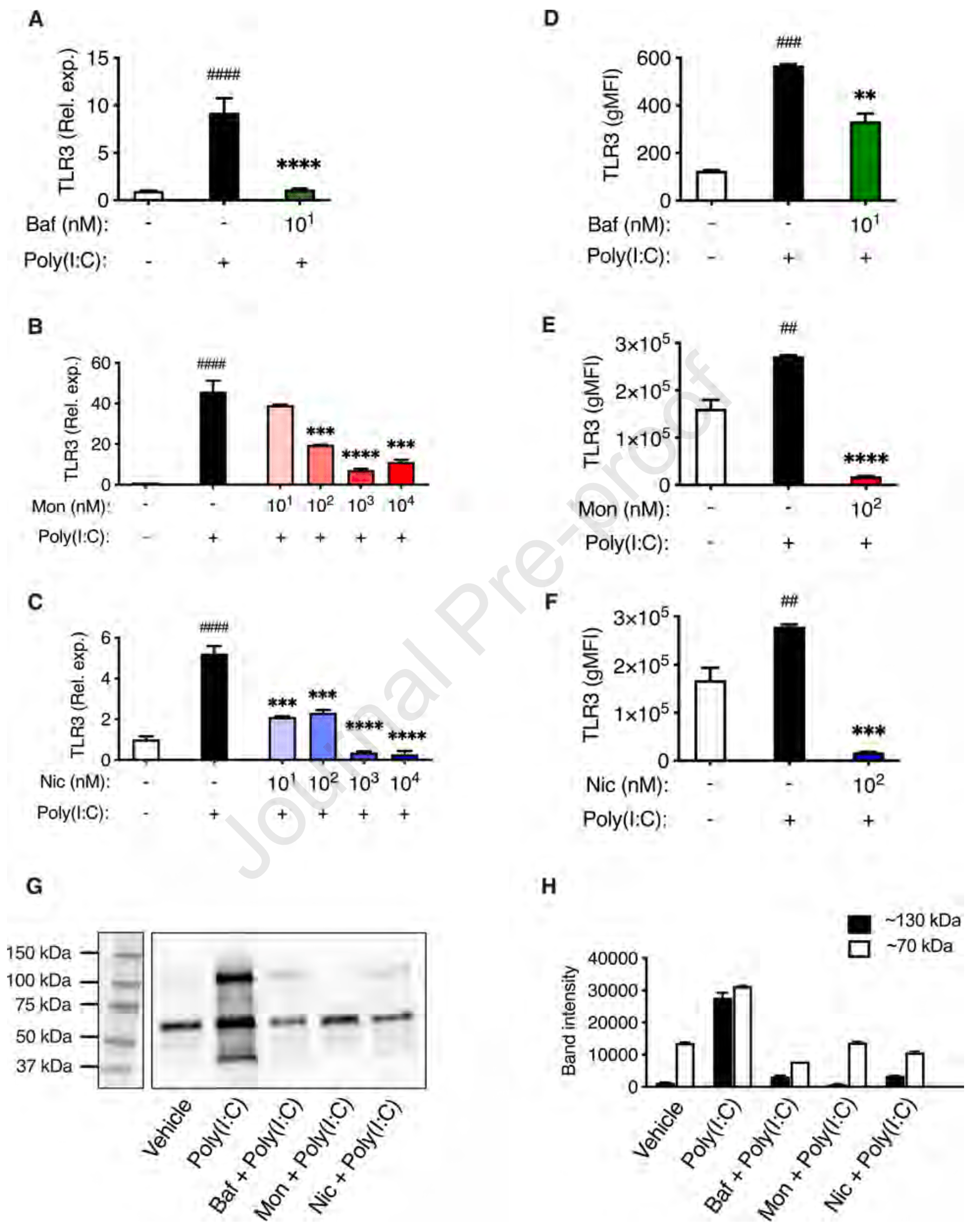
Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells
PhD # Gunnar Pejler, MSc # Xinran O Zhao, BSc Ella Fagerström, PhD Aida Paivandy
Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031
Background: Endolysosomal compartments are acidic and contain low pH-dependent proteases, and these conditions are exploited by respiratory viruses, such as SARS-CoV-2 and influenza virus, for escaping into the cytosol. Moreover, endolysosomes contain various pattern recognition receptors (PRRs), which respond to virus-derived pathogen-associated molecular patterns (PAMPs) by production of pro-inflammatory cytokines/chemokines. However, excessive pro-inflammatory responses can lead to a potentially lethal cytokine storm. Objectives: Here we investigated the endosomal PRR expression profile in primary human small airway epithelial cells (HSAECs), and whether blockade of endolysosomal acidification affects their cytokine/chemokine production after challenge with virus-derived stimulants. Methods: HSAECs were exposed to stimulants mimicking virus-derived PAMPs, either in the absence or presence of compounds causing blockade of endolysosomal acidification, followed by measurement of cytokine expression and release. Results: We show that toll-like receptor 3 (TLR3) is the major endosomal PRR expressed by HSAECs, and that TLR3 expression is strongly induced by TLR3 agonists, but not by a range of other PRR agonists. We also demonstrate that TLR3 engagement with its agonists elicits a robust pro-inflammatory cytokine/chemokine response, which is profoundly suppressed through blockade of endolysosomal acidification, by bafilomycin A1, monensin, or niclosamide. Using TLR3 reporter cells, it was confirmed that TLR3 signaling is strongly induced by Poly(I:C) and that blockade of endolysosomal acidification efficiently blocked TLR3 signaling. Finally, we show that blockade of endolysosomal acidification causes a reduction in the levels of TLR3 mRNA and protein.
Conclusion: These findings show that blockade of endolysosomal acidification suppresses TLR3-dependent cytokine and chemokine production in HSAECs. Clinical implication: These findings may be exploited for therapeutic strategies aiming to ameliorate the cytokine storm in response to respiratory virus infection. Capsule summary. This study shows that blockade of endolysosomal acidification in human airway epithelial cells causes reduced TLR3 signaling and reduced output of proinflammatory cytokines and chemokines.
References
Bird, Trapani, Villadangos, Endolysosomal proteases and their inhibitors in immunity, Nat Rev Immunol
Blasius, Beutler, Intracellular toll-like receptors, Immunity
Bortolotti, Gentili, Rizzo, Schiuma, Beltrami et al., TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection, Microorganisms
Botos, Liu, Wang, Segal, Davies, The toll-like receptor 3:dsRNA signaling complex, Biochim Biophys Acta
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae, PLoS One
Cairns, Boorgu, Levin, Kaplan, Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells, Biol Open
Cario, Podolsky, Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease, Infect Immun
Dai, Wang, Wang, Gao, Wang et al., Toll-Like Receptor Signaling in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Innate Immune Responses and the Potential Application Value of Toll-Like Receptor Immunomodulators in Patients With Coronavirus Disease 2019, Front Microbiol
Dauletbaev, Cammisano, Herscovitch, Lands, Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-beta in Airway Epithelial Cells with Minimal Costimulation of IL-8, J Immunol
Ditzel, Schwartz, Worm cure without tears. The effect of niclosamide on taeniasis saginata in man, Acta Med Scand
Edinger, Pohl, Yanguez, Stertz, Cathepsin W Is Required for Escape of Influenza A Virus from Late Endosomes, mBio
Ewald, Engel, Lee, Wang, Bogyo et al., Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase, J Exp Med
Forgac, Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology, Nat Rev Mol Cell Biol
Garcia-Cattaneo, Gobert, Muller, Toscano, Flores et al., Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling, Proc Natl Acad Sci U S A
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nat Commun
Goffic, Pothlichet, Vitour, Fujita, Meurs et al., Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells, J Immunol
Herold, Jurinovic, Arnreich, Lipworth, Hellmuth et al., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J Allergy Clin Immunol
Herrera-Rodriguez, Signorazzi, Holtrop, De Vries-Idema, Huckriede, Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production, Vaccine
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med
Ishii, Funami, Tatematsu, Seya, Matsumoto, Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells, J Immunol
J O U R N A L P R E, None
J O U R N A L P R E, None
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Jarver, Dondalska, Poux, Sandberg, Bergenstrahle et al., Single-Stranded Nucleic Acids Regulate TLR3/4/7 Activation through Interference with Clathrin-Mediated Endocytosis, Sci Rep
Jung, Nam, Oh, Jun, Ro et al., Neutralization of Acidic Intracellular Vesicles by Niclosamide Inhibits Multiple Steps of the Dengue Virus Life Cycle In Vitro, Sci Rep
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog
Kao, Huangfu, Tsai, Ho, Jhan et al., The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR, PLoS Negl Trop Dis
Kawai, Akira, Toll-like receptor and RIG-I-like receptor signaling, Ann N Y Acad Sci
Kawasaki, Kawai, Toll-like receptor signaling pathways, Front Immunol
Kircheis, Haasbach, Lueftenegger, Heyken, Ocker et al., NF-kappaB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients, Front Immunol
Ko, Cha, Lee, Bae, Ham et al., A novel defined TLR3 agonist as an effective vaccine adjuvant, Front Immunol
Leonard, Ghirlando, Askins, Bell, Margulies et al., The TLR3 signaling complex forms by cooperative receptor dimerization, Proc Natl Acad Sci U S A
Maccarana, Liu, Lampinen, Rollman, Adner et al., Monensin induces selective mast cell apoptosis through a secretory granule-mediated pathway, Allergy
Malik, Zhou, Innate Immune Sensing of Influenza A Virus, Viruses
Matsumoto, Oshiumi, Seya, Antiviral responses induced by the TLR3 pathway, Rev Med Virol
Misinzo, Delputte, Nauwynck, Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells, J Virol
Mollenhauer, Morre, Rowe, Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity, Biochim Biophys Acta
Murer, Petkidis, Vallet, Vignuzzi, Greber, Chemical Evolution of Rhinovirus Identifies Capsid-Destabilizing Mutations Driving Low-pH-Independent Genome Uncoating, J Virol
Nakamura, Funami, Komori, Yokoyama, Aiba et al., Increased expression of Toll-like receptor 3 in intrahepatic biliary epithelial cells at sites of ductular reaction in diseased livers, Hepatol Int
Naumann, Wehner, Schwarze, Petzold, Schmitz et al., Activation of dendritic cells by the novel Toll-like receptor 3 agonist RGC100, Clin Dev Immunol
Niimi, Asano, Shiraishi, Nakajima, Wakaki et al., TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA, J Immunol
Prabhakara, Godbole, Sil, Jahnavi, Gulzar et al., Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors, PLoS Pathog
Qi, Singh, Kao, Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization, J Biol Chem
Ritter, Mennerich, Weith, Seither, Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response, J Inflamm (Lond)
Sha, Truong-Tran, Plitt, Beck, Schleimer, Activation of airway epithelial cells by toll-like receptor agonists, Am J Respir Cell Mol Biol
Shang, Zhuang, Zhang, Li, Zhu et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virol J
Singh, Weiss, Goodman, Fisk, Kulkarni et al., Niclosamide-A promising treatment for COVID-19, Br J Pharmacol
Suzuki, Yamaya, Sekizawa, Hosoda, Yamada et al., Bafilomycin A(1) inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1, Am J Physiol Lung Cell Mol Physiol
Toscano, Estornes, Virard, Garcia-Cattaneo, Pierrot et al., Cleaved/associated TLR3 represents the primary form of the signaling receptor, J Immunol
Valle, Kim-Schulze, Huang, Beckmann, Nirenberg et al., An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med
Weiss, Touret, Baronti, Gilles, Hoen et al., Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2), PLoS One
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J Med Chem
Wu, Jan, Chen, Hsieh, Hwang et al., Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob Agents Chemother
Yamaya, Deng, Kikuchi, Sugawara, Saito et al., The proton ATPase inhibitor bafilomycin A1 reduces the release of rhinovirus C and cytokines from primary cultures of human nasal epithelial cells, Virus Res
Yang, Peng, Hsu, Lee, Wu et al., Repurposing old drugs as antiviral agents for coronaviruses, Biomed J
Yeganeh, Ghavami, Kroeker, Mahood, Stelmack et al., Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1, Am J Physiol Lung Cell Mol Physiol
Zhao, Lampinen, Rollman, Sommerhoff, Paivandy et al., Mast cell chymase affects the functional properties of primary human airway fibroblasts: Implications for asthma, J Allergy Clin Immunol
Zhao, Meng, Peng, Lam, Zhang et al., Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants, Emerg Microbes Infect
Zhao, Sommerhoff, Paivandy, Pejler, Mast cell chymase regulates extracellular matrix remodeling-related events in primary human small airway epithelial cells, J Allergy Clin Immunol
Zhao, To, Sze, Yung, Bian et al., A broad-spectrum virus-and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2, Nat Commun
DOI record:
{
"DOI": "10.1016/j.jaci.2024.05.031",
"ISSN": [
"0091-6749"
],
"URL": "http://dx.doi.org/10.1016/j.jaci.2024.05.031",
"alternative-id": [
"S0091674924006079"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Allergy and Clinical Immunology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jaci.2024.05.031"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology."
}
],
"author": [
{
"affiliation": [],
"family": "Pejler",
"given": "Gunnar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Xinran O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fagerström",
"given": "Ella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paivandy",
"given": "Aida",
"sequence": "additional"
}
],
"container-title": "Journal of Allergy and Clinical Immunology",
"container-title-short": "Journal of Allergy and Clinical Immunology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"jacionline.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
19
]
],
"date-time": "2024-06-19T23:16:27Z",
"timestamp": 1718838987000
},
"deposited": {
"date-parts": [
[
2024,
6,
21
]
],
"date-time": "2024-06-21T01:22:28Z",
"timestamp": 1718932948000
},
"funder": [
{
"DOI": "10.13039/100007436",
"doi-asserted-by": "publisher",
"name": "Erling Persson Family Foundation"
},
{
"DOI": "10.13039/501100002794",
"doi-asserted-by": "publisher",
"name": "Cancerfonden"
},
{
"DOI": "10.13039/501100004359",
"doi-asserted-by": "publisher",
"name": "Vetenskapsradet"
},
{
"DOI": "10.13039/501100004063",
"doi-asserted-by": "publisher",
"name": "Knut Och Alice Wallenbergs Stiftelse"
},
{
"DOI": "10.13039/501100003793",
"doi-asserted-by": "publisher",
"name": "Hjärt-lungfonden"
}
],
"indexed": {
"date-parts": [
[
2024,
6,
22
]
],
"date-time": "2024-06-22T00:25:36Z",
"timestamp": 1719015936169
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 16,
"start": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T00:00:00Z",
"timestamp": 1718582400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0091674924006079?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0091674924006079?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
6
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0091674924006079"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}