Synergistic and Antagonistic Drug Combinations against SARS-CoV-2
Tesia Bobrowski, Lu Chen, Richard T Eastman, Zina Itkin, Paul Shinn, Catherine Z Chen, Hui Guo, Wei Zheng, Sam Michael, Anton Simeonov, Matthew D Hall, Alexey V Zakharov, Eugene N Muratov
Molecular Therapy, doi:10.1016/j.ymthe.2020.12.016
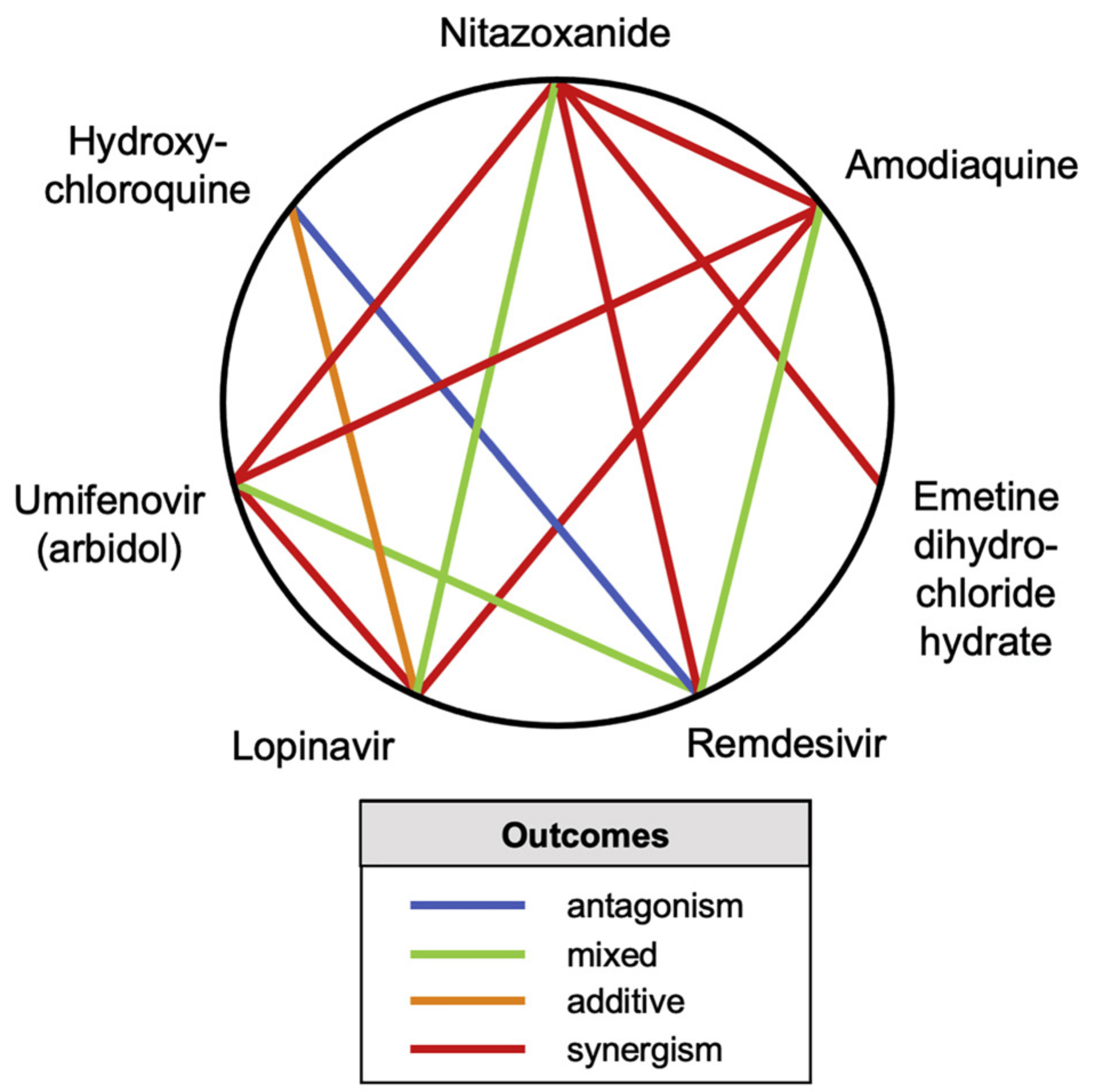
Antiviral drug development for coronavirus disease 2019 (COVID-19) is occurring at an unprecedented pace, yet there are still limited therapeutic options for treating this disease. We hypothesized that combining drugs with independent mechanisms of action could result in synergy against SARS-CoV-2, thus generating better antiviral efficacy. Using in silico approaches, we prioritized 73 combinations of 32 drugs with potential activity against SARS-CoV-2 and then tested them in vitro. Sixteen synergistic and eight antagonistic combinations were identified; among 16 synergistic cases, combinations of the US Food and Drug Administration (FDA)-approved drug nitazoxanide with remdesivir, amodiaquine, or umifenovir were most notable, all exhibiting significant synergy against SARS-CoV-2 in a cell model. However, the combination of remdesivir and lysosomotropic drugs, such as hydroxychloroquine, demonstrated strong antagonism. Overall, these results highlight the utility of drug repurposing and preclinical testing of drug combinations for discovering potential therapies to treat COVID-19.
SUPPLEMENTAL INFORMATION Supplemental Information can be found online at https://doi.org/10. 1016/j.ymthe.2020.12.016 .
ACKNOWLEDGMENTS Data-mining tools used in this study were developed under the Biomedical Data Translator Initiative of the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (grants OT3TR002020 and OT2R002514) and under support of the NIH (grant 1U01CA207160). This research was also supported by the Intramural Research Programs of the National Center for Advancing Translational Sciences (NCATS), NIH, United States.
DECLARATION OF INTERESTS The authors declare no competing interests.
References
Alves, Bobrowski, Melo-Filho, Korn, Auerbach et al., QSAR modeling of SARS-CoV Mpro inhibitors identifies Sufugolix, Cenicriviroc, Proglumetacin and other drugs as candidates for repurposing against SARS-CoV-2, Mol. Inform,
doi:10.1002/minf.202000113Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Preliminary Report, N. Engl. J. Med
Bizon, Cox, Balhoff, Kebede, Wang et al., ROBOKOP KG and KGB: Integrated Knowledge Graphs from Federated Sources, J. Chem. Inf. Model
Brimacombe, Zhao, Eastman, Hu, Wang et al., An OpenData portal to share COVID-19 drug repurposing data in real time, bioRxiv,
doi:10.1101/2020.06.04.135046Bulusu, Guha, Mason, Lewis, Muratov et al., Modelling of compound combination effects and applications to efficacy and toxicity: state-of-the-art, challenges and perspectives, Drug Discov. Today
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N. Engl. J. Med
Capuzzi, Thornton, Liu, Baker, Lam et al., Chemotext: A Publicly Available Web Server for Mining Drug-Target-Disease Relationships in PubMed, J. Chem. Inf. Model
Cherkasov, Muratov, Fourches, Varnek, Baskin et al., QSAR modeling: where have you been? Where are you going to?, J. Med. Chem
Chou, Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies, Pharmacol. Rev
Choudhary, Silakari, Scaffold morphing of arbidol (umifenovir) in search of multi-targeting therapy halting the interaction of SARS-CoV-2 with ACE2 and other proteases involved in COVID-19, Virus Res
Davidson, Williamson, Lewis, Shoemark, Carroll et al., Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein, Genome Med
Deng, Zhong, Yu, Zhang, Wang et al., Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans, Antimicrob. Agents Chemother
Eastman, Roth, Brimacombe, Simeonov, Shen et al., Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19, ACS Cent. Sci
Einav, Sobol, Gehrig, Glenn, The hepatitis C virus (HCV) NS4B RNA binding inhibitor clemizole is highly synergistic with HCV protease inhibitors, J. Infect. Dis
Ferner, Aronson, Chloroquine and hydroxychloroquine in covid-19, BMJ
Foucquier, Guedj, Analysis of drug combinations: current methodological landscape, Pharmacol. Res. Perspect
Fourches, Muratov, Tropsha, Curation of chemogenomics data, Nat. Chem. Biol
Fourches, Muratov, Tropsha, Trust, but Verify II: A Practical Guide to Chemogenomics Data Curation, J. Chem. Inf. Model
Fourches, Muratov, Tropsha, Trust, but verify: on the importance of chemical structure curation in cheminformatics and QSAR modeling research, J. Chem. Inf. Model
Glaumann, Motakefi, Jansson, Intracellular distribution and effect of the antimalarial drug mefloquine on lysosomes of rat liver, Liver
Golbraikh, Tropsha, Beware of q2!, J. Mol. Graph. Model
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Hung, Lung, Tso, Liu, Chung et al., Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Jasenosky, Cadena, Mire, Borisevich, Haridas et al., The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus, iScience
Johnson, Xie, Kalveram, Lokugamage, Muruato et al., Furin Cleavage Site Is Key to SARS-CoV-2 Pathogenesis, bioRxiv,
doi:10.1101/2020.08.26.268854Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects, PLoS Pathog
Klimstra, Tilston-Lunel, Nambulli, Boslett, Mcmillen et al., SARS-CoV-2 growth, furin-cleavage-site adaptation and neutralization using serum from acutely infected hospitalized COVID-19 patients, J. Gen. Virol
Ko, Jeon, Ryu, Kim, Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate, bioRxiv,
doi:10.1101/2020.05.12.090035Menden, Wang, Mason, Szalai, Bulusu et al., Community assessment to advance computational prediction of cancer drug combinations in a pharmacogenomic screen, Nat. Commun
Morton, Wang, Bizon, Cox, Balhoff et al., ROBOKOP: an abstraction layer and user interface for knowledge graphs to support question answering, Bioinformatics
Murakami, Wang, Babusis, Lepist, Sauer et al., Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620, Antimicrob. Agents Chemother
Muratov, Bajorath, Sheridan, Tetko, Filimonov et al., QSAR without borders, Chem. Soc. Rev
Muratov, Varlamova, Artemenko, Polishchuk, Kuz'min, Existing and Developing Approaches for QSAR Analysis of Mixtures, Mol. Inform
Rajoli, Pertinez, Arshad, Box, Tatham et al., Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, medRxiv,
doi:10.1101/2020.05.01.20087130Richards, Schwartz, Honeywell, Stewart, Cruz-Gordillo et al., Drug antagonism and single-agent dominance result from differences in death kinetics, Nat. Chem. Biol
Riva, Yuan, Yin, Martin-Sancho, Matsunaga et al., Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature
Rocco, Silva, Cruz, Junior, Tierno et al., Early use of nitazoxanide in mild Covid-19 disease: randomized, placebo-controlled trial, medRxiv,
doi:10.1101/2020.10.21.20217208Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, J. Infect. Public Health
Stockis, Allemon, De Bruyn, Gengler, Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses, Int. J. Clin. Pharmacol. Ther
Sun, He, Martínez-Romero, Kouznetsova, Tawa et al., Synergistic drug combination effectively blocks Ebola virus infection, Antiviral Res
Sun, Vilar, Tatonetti, High-Throughput Methods for Combinatorial Drug Discovery, Sci. Transl. Med
Tropsha, Best Practices for QSAR Model Development, Validation, and Exploitation, Mol. Inform
Vankadari, Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein, Int. J. Antimicrob. Agents
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Zakharov, Varlamova, Lagunin, Dmitriev, Muratov et al., QSAR Modeling and Prediction of Drug-Drug Interactions, Mol. Pharm
Zakharov, Zhao, Nguyen, Peryea, Sheils et al., Novel Consensus Architecture To Improve Performance of Large-Scale Multitask Deep Learning QSAR Models, J. Chem. Inf. Model
DOI record:
{
"DOI": "10.1016/j.ymthe.2020.12.016",
"ISSN": [
"1525-0016"
],
"URL": "http://dx.doi.org/10.1016/j.ymthe.2020.12.016",
"alternative-id": [
"S1525001620306730"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Synergistic and Antagonistic Drug Combinations against SARS-CoV-2"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Molecular Therapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ymthe.2020.12.016"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 The American Society of Gene and Cell Therapy."
}
],
"author": [
{
"affiliation": [],
"family": "Bobrowski",
"given": "Tesia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Chen",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eastman",
"given": "Richard T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Itkin",
"given": "Zina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinn",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Catherine Z.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michael",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simeonov",
"given": "Anton",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hall",
"given": "Matthew D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zakharov",
"given": "Alexey V.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4616-7036",
"affiliation": [],
"authenticated-orcid": false,
"family": "Muratov",
"given": "Eugene N.",
"sequence": "additional"
}
],
"container-title": "Molecular Therapy",
"container-title-short": "Molecular Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
12,
16
]
],
"date-time": "2020-12-16T11:00:26Z",
"timestamp": 1608116426000
},
"deposited": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T20:47:56Z",
"timestamp": 1643921276000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"1U01CA207160",
"OT2R002514",
"OT3TR002020"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000002",
"id-type": "DOI"
}
],
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/100006108",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100006108",
"id-type": "DOI"
}
],
"name": "National Center for Advancing Translational Sciences"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T02:46:08Z",
"timestamp": 1740105968407,
"version": "3.37.3"
},
"is-referenced-by-count": 85,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T00:00:00Z",
"timestamp": 1612137600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 367,
"start": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T00:00:00Z",
"timestamp": 1643846400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1525001620306730?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1525001620306730?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "873-885",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
2
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1086/653080",
"article-title": "The hepatitis C virus (HCV) NS4B RNA binding inhibitor clemizole is highly synergistic with HCV protease inhibitors",
"author": "Einav",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.ymthe.2020.12.016_bib1",
"volume": "202",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2016.11.017",
"article-title": "Synergistic drug combination effectively blocks Ebola virus infection",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.ymthe.2020.12.016_bib2",
"volume": "137",
"year": "2017"
},
{
"DOI": "10.1124/pr.58.3.10",
"article-title": "Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies",
"author": "Chou",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "Pharmacol. Rev.",
"key": "10.1016/j.ymthe.2020.12.016_bib3",
"volume": "58",
"year": "2006"
},
{
"DOI": "10.1126/scitranslmed.3006667",
"article-title": "High-Throughput Methods for Combinatorial Drug Discovery",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "205rv1",
"journal-title": "Sci. Transl. Med",
"key": "10.1016/j.ymthe.2020.12.016_bib4",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1016/j.virusres.2020.198146",
"article-title": "Scaffold morphing of arbidol (umifenovir) in search of multi-targeting therapy halting the interaction of SARS-CoV-2 with ACE2 and other proteases involved in COVID-19",
"author": "Choudhary",
"doi-asserted-by": "crossref",
"first-page": "198146",
"journal-title": "Virus Res.",
"key": "10.1016/j.ymthe.2020.12.016_bib5",
"volume": "289",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105998",
"article-title": "Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein",
"author": "Vankadari",
"doi-asserted-by": "crossref",
"first-page": "105998",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "10.1016/j.ymthe.2020.12.016_bib6",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "10.1016/j.ymthe.2020.12.016_bib7",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1038/s41467-019-09799-2",
"article-title": "Community assessment to advance computational prediction of cancer drug combinations in a pharmacogenomic screen",
"author": "Menden",
"doi-asserted-by": "crossref",
"first-page": "2674",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.ymthe.2020.12.016_bib8",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"journal-title": "Lancet",
"key": "10.1016/j.ymthe.2020.12.016_bib9",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.ymthe.2020.12.016_bib10",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19—Preliminary Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N. Engl. J. Med",
"key": "10.1016/j.ymthe.2020.12.016_bib11",
"volume": "383",
"year": "2020"
},
{
"article-title": "Viribus Unitis: Drug Combinations as a Treatment Against COVID-19",
"author": "Muratov",
"journal-title": "chemRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib12",
"year": "2020"
},
{
"DOI": "10.1038/s41589-020-0510-4",
"article-title": "Drug antagonism and single-agent dominance result from differences in death kinetics",
"author": "Richards",
"doi-asserted-by": "crossref",
"first-page": "791",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.ymthe.2020.12.016_bib13",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1111/j.1600-0676.1992.tb01045.x",
"article-title": "Intracellular distribution and effect of the antimalarial drug mefloquine on lysosomes of rat liver",
"author": "Glaumann",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "Liver",
"key": "10.1016/j.ymthe.2020.12.016_bib14",
"volume": "12",
"year": "1992"
},
{
"DOI": "10.1021/acscentsci.0c00489",
"article-title": "Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19",
"author": "Eastman",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "ACS Cent. Sci.",
"key": "10.1016/j.ymthe.2020.12.016_bib15",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1128/AAC.02350-13",
"article-title": "Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620",
"author": "Murakami",
"doi-asserted-by": "crossref",
"first-page": "1943",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.ymthe.2020.12.016_bib16",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1136/bmj.m1432",
"article-title": "Chloroquine and hydroxychloroquine in covid-19",
"author": "Ferner",
"doi-asserted-by": "crossref",
"first-page": "m1432",
"journal-title": "BMJ",
"key": "10.1016/j.ymthe.2020.12.016_bib17",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"article-title": "Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "227",
"journal-title": "J. Infect. Public Health",
"key": "10.1016/j.ymthe.2020.12.016_bib18",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "10.1016/j.ymthe.2020.12.016_bib19",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2019.07.003",
"article-title": "The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus",
"author": "Jasenosky",
"doi-asserted-by": "crossref",
"first-page": "1279",
"journal-title": "iScience",
"key": "10.1016/j.ymthe.2020.12.016_bib20",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1371/journal.ppat.1002976",
"article-title": "Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects",
"author": "Jurgeit",
"doi-asserted-by": "crossref",
"first-page": "e1002976",
"journal-title": "PLoS Pathog",
"key": "10.1016/j.ymthe.2020.12.016_bib21",
"volume": "8",
"year": "2012"
},
{
"article-title": "Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis",
"author": "Rajoli",
"journal-title": "medRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib22",
"year": "2020"
},
{
"DOI": "10.5414/CPP40213",
"article-title": "Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses",
"author": "Stockis",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "Int. J. Clin. Pharmacol. Ther.",
"key": "10.1016/j.ymthe.2020.12.016_bib23",
"volume": "40",
"year": "2002"
},
{
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomized, placebo-controlled trial",
"author": "Rocco",
"journal-title": "medRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib24",
"year": "2020"
},
{
"article-title": "Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate",
"author": "Ko",
"journal-title": "bioRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib25",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2577-1",
"article-title": "Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing",
"author": "Riva",
"doi-asserted-by": "crossref",
"first-page": "113",
"journal-title": "Nature",
"key": "10.1016/j.ymthe.2020.12.016_bib26",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1186/s13073-020-00763-0",
"article-title": "Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein",
"author": "Davidson",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Genome Med.",
"key": "10.1016/j.ymthe.2020.12.016_bib27",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1099/jgv.0.001481",
"article-title": "SARS-CoV-2 growth, furin-cleavage-site adaptation and neutralization using serum from acutely infected hospitalized COVID-19 patients",
"author": "Klimstra",
"doi-asserted-by": "crossref",
"first-page": "1156",
"journal-title": "J. Gen. Virol.",
"key": "10.1016/j.ymthe.2020.12.016_bib28",
"volume": "101",
"year": "2020"
},
{
"article-title": "Furin Cleavage Site Is Key to SARS-CoV-2 Pathogenesis",
"author": "Johnson",
"journal-title": "bioRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib29",
"year": "2020"
},
{
"DOI": "10.1128/AAC.02282-12",
"article-title": "Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "1743",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.ymthe.2020.12.016_bib30",
"volume": "57",
"year": "2013"
},
{
"DOI": "10.1021/acs.jcim.7b00589",
"article-title": "Chemotext: A Publicly Available Web Server for Mining Drug-Target-Disease Relationships in PubMed",
"author": "Capuzzi",
"doi-asserted-by": "crossref",
"first-page": "212",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib31",
"volume": "58",
"year": "2018"
},
{
"DOI": "10.1021/acs.jcim.9b00683",
"article-title": "ROBOKOP KG and KGB: Integrated Knowledge Graphs from Federated Sources",
"author": "Bizon",
"doi-asserted-by": "crossref",
"first-page": "4968",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib32",
"volume": "59",
"year": "2019"
},
{
"article-title": "COVID-KOP: Integrating Emerging COVID-19 Data with the ROBOKOP Database",
"author": "Korn",
"journal-title": "chemRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib33",
"year": "2020"
},
{
"DOI": "10.1002/minf.201000061",
"article-title": "Best Practices for QSAR Model Development, Validation, and Exploitation",
"author": "Tropsha",
"doi-asserted-by": "crossref",
"first-page": "476",
"journal-title": "Mol. Inform.",
"key": "10.1016/j.ymthe.2020.12.016_bib34",
"volume": "29",
"year": "2010"
},
{
"DOI": "10.1016/j.drudis.2015.09.003",
"article-title": "Modelling of compound combination effects and applications to efficacy and toxicity: state-of-the-art, challenges and perspectives",
"author": "Bulusu",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Drug Discov. Today",
"key": "10.1016/j.ymthe.2020.12.016_bib35",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.1021/acs.molpharmaceut.5b00762",
"article-title": "QSAR Modeling and Prediction of Drug-Drug Interactions",
"author": "Zakharov",
"doi-asserted-by": "crossref",
"first-page": "545",
"journal-title": "Mol. Pharm.",
"key": "10.1016/j.ymthe.2020.12.016_bib36",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1093/bioinformatics/btz604",
"article-title": "ROBOKOP: an abstraction layer and user interface for knowledge graphs to support question answering",
"author": "Morton",
"doi-asserted-by": "crossref",
"first-page": "5382",
"journal-title": "Bioinformatics",
"key": "10.1016/j.ymthe.2020.12.016_bib37",
"volume": "35",
"year": "2019"
},
{
"DOI": "10.1111/cts.12592",
"article-title": "The Biomedical Data Translator Program: Conception, Culture, and Community",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Clin. Transl. Sci.",
"key": "10.1016/j.ymthe.2020.12.016_bib38",
"volume": "12",
"year": "2019"
},
{
"key": "10.1016/j.ymthe.2020.12.016_bib39",
"series-title": "An AI challenge with AI2, CZI, MSR, Georgetown, NIH, and the White House. Kaggle",
"year": "2020"
},
{
"DOI": "10.1021/acs.jcim.9b00526",
"article-title": "Novel Consensus Architecture To Improve Performance of Large-Scale Multitask Deep Learning QSAR Models",
"author": "Zakharov",
"doi-asserted-by": "crossref",
"first-page": "4613",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib40",
"volume": "59",
"year": "2019"
},
{
"article-title": "QSAR modeling of SARS-CoV Mpro inhibitors identifies Sufugolix, Cenicriviroc, Proglumetacin and other drugs as candidates for repurposing against SARS-CoV-2",
"author": "Alves",
"journal-title": "Mol. Inform",
"key": "10.1016/j.ymthe.2020.12.016_bib41",
"year": "2020"
},
{
"DOI": "10.1039/D0CS00098A",
"article-title": "QSAR without borders",
"author": "Muratov",
"doi-asserted-by": "crossref",
"first-page": "3525",
"journal-title": "Chem. Soc. Rev.",
"key": "10.1016/j.ymthe.2020.12.016_bib42",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1021/jm4004285",
"article-title": "QSAR modeling: where have you been? Where are you going to?",
"author": "Cherkasov",
"doi-asserted-by": "crossref",
"first-page": "4977",
"journal-title": "J. Med. Chem.",
"key": "10.1016/j.ymthe.2020.12.016_bib43",
"volume": "57",
"year": "2014"
},
{
"DOI": "10.1021/ci100176x",
"article-title": "Trust, but verify: on the importance of chemical structure curation in cheminformatics and QSAR modeling research",
"author": "Fourches",
"doi-asserted-by": "crossref",
"first-page": "1189",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib44",
"volume": "50",
"year": "2010"
},
{
"DOI": "10.1021/acs.jcim.6b00129",
"article-title": "Trust, but Verify II: A Practical Guide to Chemogenomics Data Curation",
"author": "Fourches",
"doi-asserted-by": "crossref",
"first-page": "1243",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib45",
"volume": "56",
"year": "2016"
},
{
"DOI": "10.1038/nchembio.1881",
"article-title": "Curation of chemogenomics data",
"author": "Fourches",
"doi-asserted-by": "crossref",
"first-page": "535",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.ymthe.2020.12.016_bib46",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1016/S1093-3263(01)00123-1",
"article-title": "Beware of q2!",
"author": "Golbraikh",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "J. Mol. Graph. Model.",
"key": "10.1016/j.ymthe.2020.12.016_bib47",
"volume": "20",
"year": "2002"
},
{
"DOI": "10.1002/minf.201100129",
"article-title": "Existing and Developing Approaches for QSAR Analysis of Mixtures",
"author": "Muratov",
"doi-asserted-by": "crossref",
"first-page": "202",
"journal-title": "Mol. Inform.",
"key": "10.1016/j.ymthe.2020.12.016_bib48",
"volume": "31",
"year": "2012"
},
{
"article-title": "An OpenData portal to share COVID-19 drug repurposing data in real time",
"author": "Brimacombe",
"journal-title": "bioRxiv",
"key": "10.1016/j.ymthe.2020.12.016_bib49",
"year": "2020"
},
{
"DOI": "10.1002/prp2.149",
"article-title": "Analysis of drug combinations: current methodological landscape",
"author": "Foucquier",
"doi-asserted-by": "crossref",
"first-page": "e00149",
"journal-title": "Pharmacol. Res. Perspect.",
"key": "10.1016/j.ymthe.2020.12.016_bib50",
"volume": "3",
"year": "2015"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.06.29.178889",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1525001620306730"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Synergistic and Antagonistic Drug Combinations against SARS-CoV-2",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "29"
}