The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: an updated meta-analysis of randomized trials
Xudong Han, Wei Shi, MS. Ya Yang
Journal of Thoracic Disease, doi:10.21037/jtd-23-1043
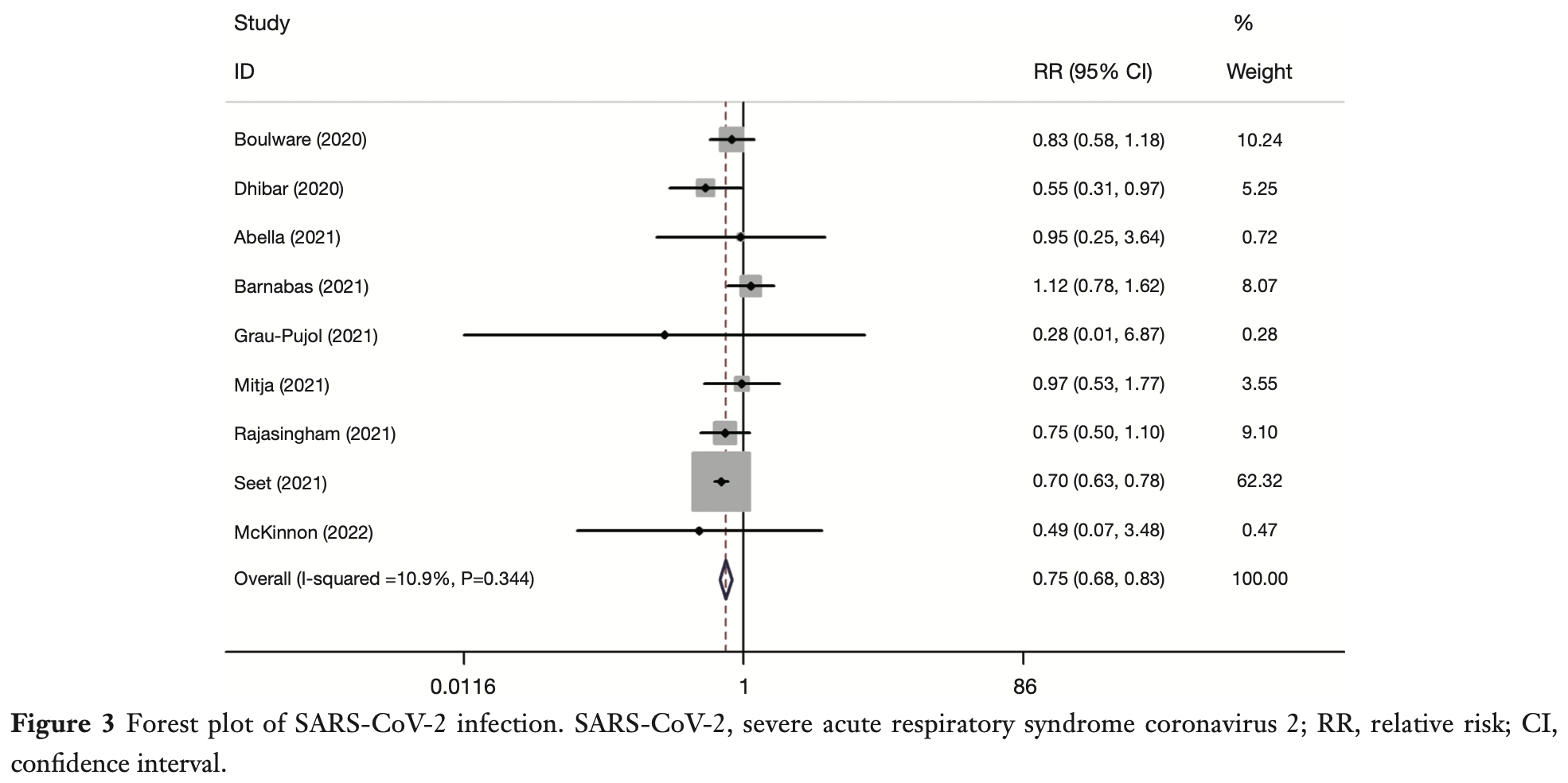
Background: Coronavirus disease 2019 (COVID-19), a disease that affected tens of millions of people, upended the lives of countless individuals around the globe. The chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) were the most frequently cited as potential treatments and preventatives against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The primary aim of this investigation was to scrutinize the effectiveness and safety of HCQ for COVID-19 prevention and to present powerful evidence and reference for clinical practice. Methods: PubMed, Ovid and the Cochrane COVID-19 Register of Controlled Trials (CENTRAL) were systematically searched from inception to January 31, 2022. Randomized controlled trials (RCTs) trials that included participants who were SARS-CoV-2 negative at the time of registration were enrolled in this metaanalysis. The intervention group took HCQ or CQ orally. The control group was not blinded by quinine or placebo. Pooled relative risk (RR) of SARS-CoV-2 infection, mortality, hospitalization, adverse events, and compliance were calculated. The software tools utilized for statistical analyses were Stata 14 and Review Manager 5.3. Results: A total of 9 studies including 7,825 participants were enrolled. Bias of individual studies were assessed as low risk. The pooled RR for SARS-CoV-2 infection was 0.75 [95% confidence interval (CI): 0.68-0.83] (z=-4.01, P<0.0001; I 2 =11%). The pooled RR for hospitalization was 0.72 (95% CI: 0.35-1.50) (z=0.87, P=0.39; I 2 =0.0%). The pooled RR for mortality and adverse events were 3.26 (95% CI: 0.13-79.74) (z=0.72, P=0.47; I 2 =0.0%) and 1.90 (95% CI: 1.20-3.02) (z=2.73, P=0.0063; I 2 =94%). Conclusions: Results of this meta-analysis indicated significant impact of HCQ on SARS-CoV-2 infection with higher risk of adverse events. These findings must be considered with caution, and further research is necessary to delineate the specific circumstances where HCQ may be effective for COVID-19 prevention.
Conflicts of Interest
References
Abella, Jolkovsky, Biney, Efficacy and Safety of Hydroxychloroquine vs Placebo for Preexposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial, JAMA Intern Med
Ather, Patel, Ruparel, Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care, J Endod
Barnabas, Brown, Bershteyn, Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Randomized Trial, Ann Intern Med
Bartoszko, Siemieniuk, Kum, Prophylaxis against covid-19: living systematic review and network meta-analysis, BMJ
Boulware, Pullen, Bangdiwala, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N Engl J Med
Cortegiani, Ingoglia, Ippolito, A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19, J Crit Care
Cumpston, Li, Page, Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Database Syst Rev
Deming, Chen, COVID-19 and Lessons to Be Learned from Prior Coronavirus Outbreaks, Ann Am Thorac Soc
Dersimonian, Laird, Meta-analysis in clinical trials revisited, Contemp Clin Trials
Dersimonian, Laird, Meta-analysis in clinical trials, Control Clin Trials
Dhibar, Arora, Kakkar, Post-exposure prophylaxis with hydroxychloroquine for the prevention of COVID-19, a myth or a reality? The PEP-CQ Study, Int J Antimicrob Agents
Egger, Smith, Schneider, Bias in meta-analysis detected by a simple, graphical test, BMJ
Faraone, Qu, Goodarzi, Immune evasion and membrane fusion of SARS-CoV-2 XBB subvariants EG.5.1 and XBB.2.3, Emerg Microbes Infect
García-Albéniz, Amo, Polo, Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, Eur J Epidemiol
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Higgins, Altman, Gøtzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ
Higgins, Thompson, Deeks, Measuring inconsistency in meta-analyses, BMJ
Hong, Friedland, Hu, Safety and efficacy of hydroxychloroquine as prophylactic against COVID-19 in healthcare workers: a meta-analysis of randomised clinical trials, BMJ Open
Jorge, Ung, Young, Hydroxychloroquine retinopathy -implications of research advances for rheumatology care, Nat Rev Rheumatol
Keyaerts, Vijgen, Maes, In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine, Biochem Biophys Res Commun
Kumar, Khodor, Pathophysiology and treatment strategies for COVID-19, J Transl Med
Lewis, Chaudhuri, Alshamsi, The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis: A systematic review and meta-analysis of randomized trials, PLoS One
Lu, Zhao, Li, Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet
Mavridis, Salanti, How to assess publication bias: funnel plot, trim-and-fill method and selection models, Evid Based Ment Health
Mckinnon, Wang, Zervos, Safety and tolerability of hydroxychloroquine in health care workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study, Int J Infect Dis
Mitjà, Corbacho-Monné, Ubals, A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19, N Engl J Med
Moher, Liberati, Tetzlaff, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Int J Surg
Rajasingham, Bangdiwala, Nicol, Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial, Clin Infect Dis
Rodrigo, Fernando, Rajapakse, Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review, Clin Microbiol Infect
Salian, Wright, Vedell, COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies, Mol Pharm
Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology, Nat Rev Rheumatol
Seet, Quek, Ooi, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int J Infect Dis
Shetty, Namachivayam, Evidence for Chloroquine/ Hydroxychloroquine in the Treatment of COVID-19, Indian J Crit Care Med
Singh, Chauhan, Kakkar, Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead, Eur J Pharmacol
Sterne, Savović, Page, RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ
Tindale, Stockdale, Coombe, Evidence for transmission of COVID-19 prior to symptom onset, Elife
Vincent, Bergeron, Benjannet, Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Weston, Coleman, Haupt, Broad Anticoronavirus Activity of Food and Drug Administration-Approved Drugs against SARS-CoV-2 In Vitro and SARS-CoV In Vivo, J Virol
Yao, Ye, Zhang, In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Zeouk, Bekhti, Lorenzo-Morales, From Wuhan to COVID-19 Pandemic: An Up-to-Date Review of Its Pathogenesis, Potential Therapeutics, and Recent Advances, Microorganisms
Zhou, Dai, Tong, COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression, J Antimicrob Chemother
Zhou, Verweij, Bijlsma, Repurposed drug studies on the primary prevention of SARS-CoV-2 infection during the pandemic: systematic review and meta-analysis, BMJ Open Respir Res
DOI record:
{
"DOI": "10.21037/jtd-23-1043",
"ISSN": [
"2072-1439",
"2077-6624"
],
"URL": "http://dx.doi.org/10.21037/jtd-23-1043",
"author": [
{
"affiliation": [],
"family": "Han",
"given": "Xudong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shi",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Ya",
"sequence": "additional"
}
],
"container-title": "Journal of Thoracic Disease",
"container-title-short": "J Thorac Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"amegroups.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
31
]
],
"date-time": "2024-05-31T01:58:33Z",
"timestamp": 1717120713000
},
"deposited": {
"date-parts": [
[
2024,
5,
31
]
],
"date-time": "2024-05-31T01:59:12Z",
"timestamp": 1717120752000
},
"indexed": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:28:10Z",
"timestamp": 1717201690423
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2024,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2024,
5
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
}
},
"link": [
{
"URL": "https://jtd.amegroups.com/article/download/86436/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8611",
"original-title": [],
"page": "2983-2993",
"prefix": "10.21037",
"published": {
"date-parts": [
[
2024,
5
]
]
},
"published-online": {
"date-parts": [
[
2024,
5
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
},
"publisher": "AME Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://jtd.amegroups.com/article/view/86436/html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: an updated meta-analysis of randomized trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.21037/ame_crossmark_policy",
"volume": "16"
}