Hydroxy Chloroquine Prophylaxis Experience in Doctor Community with COVID-19 in West Bengal
Pranab Kumar Dey, Dr Tapas Kumar Sabui, Sreetama Chowdhury, Joyashree Banerjee, Abhisek Sabui, Jashodhara Choudhuri
Journal of College of Medical Sciences-Nepal, doi:10.3126/jcmsn.v20i2.43302
Background The Indian Council of Medical Research (ICMR)National task Force for COVID-19 reviewed the data on in-vitro testing of Hydroxy Chloroquine (HCQ)for antiviral efficacy against SARS-CoV-2, safety profile and recommended it's use for prophylaxis among health care workers (HCWs). The efficacy of HCQ against Covid -19 has been the subject of contradictory results. Given the need for insights into the clinical outcomes and side effects among doctors on prophylaxis with hydroxychloroquine, we conducted a retrospective analysis of doctors treated at home or hospital with COVID-19 infection.
Methods The data was collected by direct interview, electronic data transfer from COVID-19 positive doctors using real-time reverse transcription-polymerase chain reaction (RT-PCR) test from two medical colleges, friends and relatives of doctors who voluntarily provided their illness information during the period of August, 26 to Nov 25, 2020.
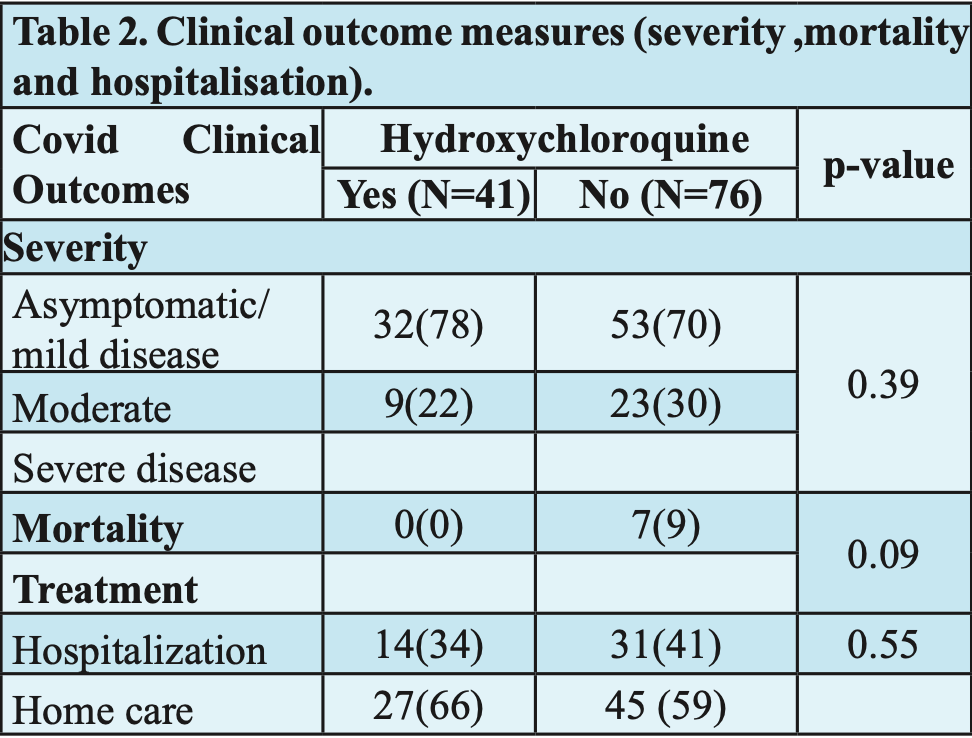
Results A total of 117 doctors participated in study. Out of 117 covid positive doctors, 76 received no HCQ prophylaxis and 41 received prophylaxis till the onset of the symptom. Out of 117 positive doctors, 32(27%) developed moderate to severe (MS) illness. Nine doctors (22%) developed MS in HCQ group and none of them died. Out of no HCQ group 23(30%) developed MS illness and 7(9%) died. There was no statistically significant relation between use of HCQ and outcomes of disease. The most common (22%) side effect of HCQ prophylaxis was related to gastrointestinal system.
Conclusions Hydroxy Chloroquine has a disease modifying effect on COVID-19 disease although it is not robust.
Conflict of interest: None
References
Abella, Jolkovsky, Biney, Efficacy and safety of hydroxychloroquinevs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial, JAMA Intern Med,
doi:10.1001/jamainternmed.2020.6319Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2020.105938Fantini, Scala, Chahinian, Yahi, Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection, IntJAntimicrob Agents,
doi:10.1016/j.ijantimicag.2020.105960Gandhi, Lynch, Del Rio, Mild or Moderate COVID-19, N Engl J Med
Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends,
doi:10.5582/bst.2020.01047Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrlert et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell,
doi:10.1016/j.cell.2020.02.052Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov,
doi:10.1038/s41421-020-0156-0Lofgren, Nicol, Bangdiwala, Pastick, Okafor et al., Safety of hydroxychloroquine among outpatient clinical trial participantsfor COVID-19, Open Forum InfectDis
Rajasingham, Bangdiwala, Nicol, Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, Nephrol Dial Transplant,
doi:10.1093/cid/ciaa1571Raphael, Stricker, Fesler, Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Workers from India:A Meta-Analysis, Journal of Infection and Public Health,
doi:10.1016/j.jiph.2021.08.001Reedac, Jessicaj, Long, Dena, Ariel et al., Drug treatments for COVID-19: living systematic review and network meta-analysis, BMJ,
doi:10.1136/bmj.m2980Rentsch, Devito, Mackenna, Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform, Lancet Rheumatol,
doi:10.1016/S2665-9913(20)30378-7Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology, Nat Rev Rheumatol,
doi:10.1038/s41584-020-0372-xDOI record:
{
"DOI": "10.3126/jcmsn.v20i2.43302",
"ISSN": [
"2091-0673",
"2091-0657"
],
"URL": "http://dx.doi.org/10.3126/jcmsn.v20i2.43302",
"abstract": "<jats:p>Background The Indian Council of Medical Research (ICMR)National task Force for COVID-19 reviewed the data on in-vitro testing of Hydroxy Chloroquine (HCQ)for antiviral efficacy against SARS-CoV-2, safety profile and recommended it’s use for prophylaxis among health care workers (HCWs). The efficacy of HCQ against Covid -19 has been the subject of contradictory results. Given the need for insights into the clinical outcomes and side effects among doctors on prophylaxis with hydroxychloroquine, we conducted a retrospective analysis of doctors treated at home or hospital with COVID-19 infection.MethodsThe data was collected by direct interview, electronic data transfer from COVID-19 positive doctors using real-time reverse transcription-polymerase chain reaction (RT-PCR) test from two medical colleges, friends and relatives of doctors who voluntarily provided their illness information during the period of August, 26 to Nov 25, 2020.ResultsA total of 117 doctors participated in study. Out of 117 covid positive doctors, 76 received no HCQ prophylaxis and 41 received prophylaxis till the onset of the symptom. Out of 117 positive doctors, 32(27%) developed moderate to severe (MS) illness. Nine doctors (22%) developed MS in HCQ group and none of them died. Out of no HCQ group 23(30%) developed MS illness and 7(9%) died. There was no statistically significant relation between use of HCQ and outcomes of disease. The most common (22%) side effect of HCQ prophylaxis was related to gastrointestinal system. Conclusions Hydroxy Chloroquine has a disease modifying effect on COVID-19 disease although it is not robust.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2356-7627",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dey",
"given": "Pranab Kumar",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0009-9074",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sabui",
"given": "Tapas Kumar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2856-4839",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chowdhury",
"given": "Sreetama",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3893-7811",
"affiliation": [],
"authenticated-orcid": false,
"family": "Banerjee",
"given": "Joyashree",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0349-2579",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sabui",
"given": "Abhisek",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0105-0937",
"affiliation": [],
"authenticated-orcid": false,
"family": "Choudhuri",
"given": "Jashodhara",
"sequence": "additional"
}
],
"container-title": "Journal of College of Medical Sciences-Nepal",
"container-title-short": "J Coll Med Sci-Nepal",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T12:18:48Z",
"timestamp": 1719836328000
},
"deposited": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T12:19:20Z",
"timestamp": 1719836360000
},
"indexed": {
"date-parts": [
[
2024,
7,
2
]
],
"date-time": "2024-07-02T00:17:13Z",
"timestamp": 1719879433937
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
6,
30
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
7,
1
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
30
]
],
"date-time": "2024-06-30T00:00:00Z",
"timestamp": 1719705600000
}
}
],
"link": [
{
"URL": "https://www.nepjol.info/index.php/JCMSN/article/download/43302/51429",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nepjol.info/index.php/JCMSN/article/download/43302/51429",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1670",
"original-title": [],
"page": "147-152",
"prefix": "10.3126",
"published": {
"date-parts": [
[
2024,
6,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
30
]
]
},
"publisher": "Nepal Journals Online (JOL)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nepjol.info/index.php/JCMSN/article/view/43302"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Hydroxy Chloroquine Prophylaxis Experience in Doctor Community with COVID-19 in West Bengal",
"type": "journal-article",
"volume": "20"
}