Can hydroxychloroquine and azithromycin combination cause cardiac rhythm disturbances in children with COVID-19 pneumonia?
Damla Geçkalan, Rahmi Özdemir, Yasin Yılmaz, Cemile Hilal Çelik, Batuhan Berk Demir, Yeşim Tunç
Journal of Health Sciences and Medicine, doi:10.32322/jhsm.1625339
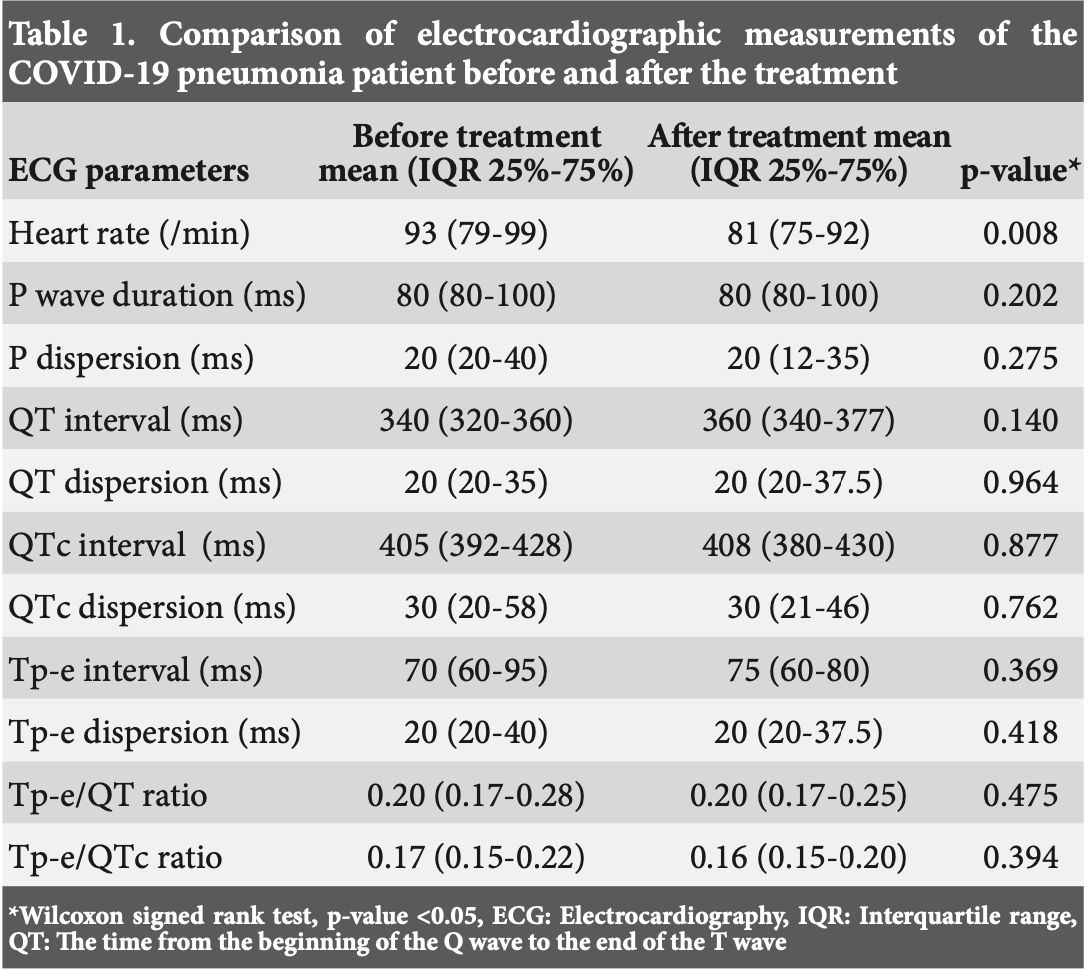
Aims: At the beginning of the COVID-19 pandemic; it has been shown that receiving hydroxychloroquine and azithromycin treatment decrease viral carriage of coronavirus in patients. In this study, we aimed to evaluate electrocardiography (ECG) abnormalities in pediatric patients with COVID-19 pneumonia receiving combined therapy with hydroxychloroquine and azithromycin. Methods: In this study; ECG and laboratory parameters of 24 children with COVID-19 pneumonia who were treated with hydroxychloroquine and azithromycin at Health Sciences University between June 2020 and November 2020 were analyzed retrospectively. P wave dispersion (PWd), QT interval (QT), QT dispersion (QTd), QTc interval (QTc), QTc dispersion (QTcd), Tpeak-Tend interval (Tp-e), Tp-e dispersion (Tp-ed), Tp-e/QT, Tp-Te/QTc ratios were evaluated with 12 lead ECG. ECG parameters and QTc interval were compared before and after (5 days) the treatment. Results: The mean age was 13±4.5 years and 62.5% were female. Median hospitalization length was 6 days. There was no statistically significant difference between the PWd, QT and QTc interval, QTd, QTcd, Tp-e interval, Tp-e dispersion, Tp-e/QT, Tp-e/QTd measurements and ratios of the before and after treatment. A significant difference was found for the decrease in hearth rate in regard to the measurement before and after the treatment. Conclusion: In our study, there were no rhythm problems which were observed on ECG in pediatric patients receiving hydroxychloroquine and azithromycin combination therapy for COVID-19 pneumonia. We also found that laboratory parameters were not specific for COVID-19 pneumonia in children.
Conflict of Interest Statement The authors have no conflicts of interest to declare.
Financial Disclosure The authors declared that this study has received no financial support.
Author Contributions All of the authors declare that they have all participated in the design, execution, and analysis of the paper, and that they have approved the final version.
References
Akkuş, Bal, Yaqoobi, Bekler, Akkuş et al., Electrocardiographic findings and cardiac safety of hydroxychloroquine +azithromycin in hospitalized patients with COVID-19, Cukurova Med J,
doi:10.17826/cumj.856174Asensio, Acunzo, Uribe, Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence, J Interv Card Electrophysiol,
doi:10.1007/s10840-020-00765-3Bebitoğlu, Oğuz, Hodzic, Oğuz, Hodzic et al., Chloroquine/hydroxychloroquine: pharmacological view of an old drug currently used in COVID-19 treatment, Anat Clin,
doi:10.21673/anadoluklin.735826Beukelman, Patkar, Saag, American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features, Arthritis Care Res,
doi:10.1002/acr.20460Braun, Ferner, Kronfeld, Griese, Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases, Pediatr Pulmonol,
doi:10.1002/ppul.23133Food&drug Adminastiration, drugs, drug safety and availability, FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems
Gautret, Hoang, Lagier, Raoult, Effect of hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, an update with an intention-to-treat analysis and clinical outcomes, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2020.106239Gautret, Honoré, Lagier, Raoult, Safety profile of hydroxychloroquine and azithromycin combined treatment in COVID-19 patients, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2020.106236Goldman, Diaz, Urba, Use of hydroxychloroquine in combination with azithromycin for patients with COVID-19 is not supported by recent literature, Int J Antimicrob Agents,
doi:10.1016/j.ijantimicag.2020.106174Hançerli Törün, Kaba, Yanartas, Plasma D-dimer: a promising indicator of COVID-19 infection severity or only an acute phase reactant, Minerva Pediatr,
doi:10.23736/S2724-5276.21.06170-XImazio, Klingel, Kindermann, COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis?, Heart,
doi:10.1136/heartjnl-2020-317186Lagier, Million, Gautret, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis, Travel Med Infect Dis,
doi:10.1016/j.tmaid.2020.101791Li, Cheng, Will Hydroxychloroquine still be a game-changer for COVID-19 by combining azithromycin?, Front Immunol,
doi:10.3389/fimmu.2020.01969Ludvigsson, Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults, Acta Paediatr,
doi:10.1111/apa.15270Mercuro, Yen, Shim, Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus disease 2019 (COVID-19), JAMA Cardiol,
doi:10.1001/jamacardio.2020.1834Morens, Taubenberger, Fauci, Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness, J Infect Dis,
doi:10.1086/591708Okour, Al-Kofahi, Hydroxychloroquine and azithromycin as potential treatments for COVID-19; clinical status impacts the outcome, J Pharmacokinet Pharmacodyn,
doi:10.1007/s10928-020-09689-xRetsema, Girard, Schelkly, Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms, Antimicrob Agents Chemother,
doi:10.1128/AAC.31.12.1939Sarayani, Cicali, Henriksen, Brown, Safety signals for QT prolongation or torsades de pointes associated with azithromycin with or without chloroquine or hydroxychloroquine, Res Social Adm Pharm,
doi:10.1016/j.sapharm.2020.04.016Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology, Nat Rev Rheumatol,
doi:10.1038/s41584-020-0372-xTisdale, Drug-induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management, Can Pharm J (Ott),
doi:10.1177/1715163516641136Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China, JAMA,
doi:10.1001/jama.2020.1585White, Miller, Churchill, Chloroquine treatment of severe malaria in children. Pharmacokinetics, toxicity, and new dosage recommendations, N Engl J Med,
doi:10.1056/NEJM198812083192301Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet,
doi:10.1016/S0140-6736(20)30566-3Zimmermann, Curtis, Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children, Pediatr Infect Dis J,
doi:10.1097/INF.0000000000002660DOI record:
{
"DOI": "10.32322/jhsm.1625339",
"ISSN": [
"2636-8579"
],
"URL": "http://dx.doi.org/10.32322/jhsm.1625339",
"abstract": "<jats:p xml:lang=\"en\">Aims: At the beginning of the COVID-19 pandemic; it has been shown that receiving hydroxychloroquine and azithromycin treatment decrease viral carriage of coronavirus in patients. In this study, we aimed to evaluate electrocardiography (ECG) abnormalities in pediatric patients with COVID-19 pneumonia receiving combined therapy with hydroxychloroquine and azithromycin.\r\nMethods: In this study; ECG and laboratory parameters of 24 children with COVID-19 pneumonia who were treated with hydroxychloroquine and azithromycin at Health Sciences University between June 2020 and November 2020 were analyzed retrospectively. P wave dispersion (PWd), QT interval (QT), QT dispersion (QTd), QTc interval (QTc), QTc dispersion (QTcd), Tpeak-Tend interval (Tp-e), Tp-e dispersion (Tp-ed), Tp-e/QT, Tp-Te/QTc ratios were evaluated with 12 lead ECG. ECG parameters and QTc interval were compared before and after (5 days) the treatment.\r\nResults: The mean age was 13±4.5 years and 62.5% were female. Median hospitalization length was 6 days. There was no statistically significant difference between the PWd, QT and QTc interval, QTd, QTcd, Tp-e interval, Tp-e dispersion, Tp-e/QT, Tp-e/QTd measurements and ratios of the before and after treatment. A significant difference was found for the decrease in hearth rate in regard to the measurement before and after the treatment. \r\nConclusion: In our study, there were no rhythm problems which were observed on ECG in pediatric patients receiving hydroxychloroquine and azithromycin combination therapy for COVID-19 pneumonia. We also found that laboratory parameters were not specific for COVID-19 pneumonia in children.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
2,
25
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0001-6344-7035",
"affiliation": [
{
"name": "Bakırçay Üniversitesi, Çiğli Eğitim ve Araştırma Hastanesi"
}
],
"authenticated-orcid": true,
"family": "Geçkalan",
"given": "Damla",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-2775-166X",
"affiliation": [
{
"name": "İZMİR KATİP ÇELEBİ ÜNİVERSİTESİ"
}
],
"authenticated-orcid": true,
"family": "Özdemir",
"given": "Rahmi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1724-9833",
"affiliation": [
{
"name": "KUTAHYA HEALTH SCIENCES UNIVERSITY"
}
],
"authenticated-orcid": true,
"family": "Yılmaz",
"given": "Yasin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8065-7082",
"affiliation": [
{
"name": "KUTAHYA HEALTH SCIENCES UNIVERSITY"
}
],
"authenticated-orcid": true,
"family": "Çelik",
"given": "Cemile Hilal",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0349-5112",
"affiliation": [
{
"name": "KUTAHYA HEALTH SCIENCES UNIVERSITY"
}
],
"authenticated-orcid": true,
"family": "Demir",
"given": "Batuhan Berk",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1078-8730",
"affiliation": [
{
"name": "KUTAHYA HEALTH SCIENCES UNIVERSITY"
}
],
"authenticated-orcid": true,
"family": "Tunç",
"given": "Yeşim",
"sequence": "additional"
}
],
"container-title": "Journal of Health Sciences and Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
26
]
],
"date-time": "2025-03-26T05:31:27Z",
"timestamp": 1742967087000
},
"deposited": {
"date-parts": [
[
2025,
3,
26
]
],
"date-time": "2025-03-26T23:22:57Z",
"timestamp": 1743031377000
},
"indexed": {
"date-parts": [
[
2025,
3,
26
]
],
"date-time": "2025-03-26T23:40:02Z",
"timestamp": 1743032402477,
"version": "3.40.3"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2025,
3,
21
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2025,
3,
21
]
]
}
},
"member": "17352",
"original-title": [],
"page": "308-312",
"prefix": "10.32322",
"published": {
"date-parts": [
[
2025,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
21
]
]
},
"publisher": "Journal of Health Sciences and Medicine",
"reference": [
{
"key": "ref1",
"unstructured": "Kannan S, Shaik Syed Ali P, Sheeza A, Hemalatha K. COVID-19 (novel Coronavirus 2019)-recent trends. Eur Rev Med Pharmacol Sci. 2020; 24(4):2006-2011. doi:10.26355/eurrev_202002_20378"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "crossref",
"key": "ref2",
"unstructured": "Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5"
},
{
"DOI": "10.5152/TurkArchPediatr.2021.21076",
"doi-asserted-by": "crossref",
"key": "ref3",
"unstructured": "Üzel VH, Yılmaz K, Şen V, et al. Evaluation of hematological parameters of children diagnosed with COVID-19: single-center experience. Turk Arch Pediatr. 2021;56(5):463-468. doi:10.5152/TurkArchPediatr.2021. 21076"
},
{
"DOI": "10.1111/apa.15270",
"doi-asserted-by": "crossref",
"key": "ref4",
"unstructured": "Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6): 1088-1095. doi:10.1111/apa.15270"
},
{
"DOI": "10.1097/INF.0000000000002660",
"doi-asserted-by": "crossref",
"key": "ref5",
"unstructured": "Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355-368. doi:10.1097/INF.0000000000002660"
},
{
"DOI": "10.1007/s10928-020-09689-x",
"doi-asserted-by": "crossref",
"key": "ref6",
"unstructured": "Okour M, Al-Kofahi M, Austin D. Hydroxychloroquine and azithromycin as potential treatments for COVID-19; clinical status impacts the outcome. J Pharmacokinet Pharmacodyn. 2020;47(3):187-188. doi:10.1007/s10928-020-09689-x"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106236",
"doi-asserted-by": "crossref",
"key": "ref7",
"unstructured": "Gautret P, Honoré S, Lagier JC, Raoult D. Safety profile of hydroxychloroquine and azithromycin combined treatment in COVID-19 patients. Int J Antimicrob Agents. 2021;57(1):106236. doi:10. 1016/j.ijantimicag.2020.106236"
},
{
"DOI": "10.1016/j.tmaid.2020.101791",
"doi-asserted-by": "crossref",
"key": "ref8",
"unstructured": "Lagier JC, Million M, Gautret P, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med Infect Dis. 2020;36:101791. doi:10.1016/j.tmaid.2020.101791"
},
{
"DOI": "10.21673/anadoluklin.735826",
"doi-asserted-by": "crossref",
"key": "ref9",
"unstructured": "Terzioğlu Bebitoğlu B, Oğuz E, Hodzic A, Oğuz E, Hodzic A, Hatiboğlu N, Kam Ö. Chloroquine/hydroxychloroquine: pharmacological view of an old drug currently used in COVID-19 treatment. Anat Clin. 2020;25(1): 204-215. doi:10.21673/anadoluklin.735826"
},
{
"DOI": "10.1007/s12016-010-8243-x",
"doi-asserted-by": "crossref",
"key": "ref10",
"unstructured": "Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145-153. doi:10.1007/s12016-010-8243-x"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106239",
"doi-asserted-by": "crossref",
"key": "ref11",
"unstructured": "Gautret P, Hoang VT, Lagier JC, Raoult D. Effect of hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, an update with an intention-to-treat analysis and clinical outcomes. Int J Antimicrob Agents. 2021;57(1): 106239. doi:10.1016/j.ijantimicag.2020.106239"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106174",
"doi-asserted-by": "crossref",
"key": "ref12",
"unstructured": "Goldman JD, Diaz G, Urba WJ. Use of hydroxychloroquine in combination with azithromycin for patients with COVID-19 is not supported by recent literature. Int J Antimicrob Agents. 2021;57(1):106174. doi:10.1016/j.ijantimicag.2020.106174"
},
{
"key": "ref13",
"unstructured": "U.S Food&Drug Adminastiration, drugs, drug safety and availability, FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-COVID-19-outside-hospital-setting-or"
},
{
"key": "ref14",
"unstructured": "Republic of Turkiye Ministry of Health, Department of Public Health, COVID-19 Guideline. 02.02.2020. Available from: https://dosyamerkez.saglik.gov.tr/Eklenti/37044/0/covid-19rehberipdf.pdf"
},
{
"key": "ref15",
"unstructured": "Republic of Turkiye Ministry of Health, Department of Public Health Guideline. 06.01.2022. Available from: https://covid19.saglik.gov.tr/Eklenti/42283/0/covid-19rehbericocukhastayonetimivetedavi06012022v1pdf.pdf"
},
{
"DOI": "10.5798/dicletip.1005406",
"doi-asserted-by": "crossref",
"key": "ref16",
"unstructured": "Aktar F, Sağır H. Pediatric COVID-19 and its approach. Dicle Med J. 2021;48(3):166-175. doi:10.5798/dicletip.1005406"
},
{
"DOI": "10.1007/s10840-020-00765-3",
"doi-asserted-by": "crossref",
"key": "ref17",
"unstructured": "Asensio E, Acunzo R, Uribe W, et al. Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence. J Interv Card Electrophysiol. 2020;59(2)315-320. doi:10.1007/s10840-020-00765-3"
},
{
"key": "ref18",
"unstructured": "World Health Organization. The cardiotoxicity of antimalarials: Malaria Policy Advisory Committee Meeting. Published March 24, 2017. Available from: https://www.who.int/malaria/mpac/mpac-mar2017- erg-cardiotoxicity-report-session2.pdf"
},
{
"DOI": "10.1001/jamacardio.2020.1834",
"doi-asserted-by": "crossref",
"key": "ref19",
"unstructured": "Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036-1041. doi:10.1001/jamacardio.2020.1834"
},
{
"DOI": "10.3389/fimmu.2020.01969",
"doi-asserted-by": "crossref",
"key": "ref20",
"unstructured": "Li C, Cheng G. Will Hydroxychloroquine still be a game-changer for COVID-19 by combining azithromycin? Front Immunol. 2020;11:1969. doi:10.3389/fimmu.2020.01969"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "crossref",
"key": "ref21",
"unstructured": "Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. doi:10.1016/S0140-6736(20) 30566-3"
},
{
"DOI": "10.1128/AAC.31.12.1939",
"doi-asserted-by": "crossref",
"key": "ref22",
"unstructured": "Retsema J, Girard A, Schelkly W, et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987;31(12):1939-1947. doi:10.1128/AAC.31.12.1939"
},
{
"DOI": "10.1086/591708",
"doi-asserted-by": "crossref",
"key": "ref23",
"unstructured": "Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962-970. doi: 10.1086/591708"
},
{
"DOI": "10.1056/NEJM198812083192301",
"doi-asserted-by": "crossref",
"key": "ref24",
"unstructured": "White NJ, Miller KD, Churchill FC, et al. Chloroquine treatment of severe malaria in children. Pharmacokinetics, toxicity, and new dosage recommendations. N Engl J Med. 1988;319(23):1493-1500. doi:10.1056/NEJM198812083192301"
},
{
"DOI": "10.1038/s41584-020-0372-x",
"doi-asserted-by": "crossref",
"key": "ref25",
"unstructured": "Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155-166. doi:10.1038/s41584-020-0372-x"
},
{
"DOI": "10.1002/acr.20460",
"doi-asserted-by": "crossref",
"key": "ref26",
"unstructured": "Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63(4):465-482. doi:10.1002/acr.20460"
},
{
"DOI": "10.1002/ppul.23133",
"doi-asserted-by": "crossref",
"key": "ref27",
"unstructured": "Braun S, Ferner M, Kronfeld K, Griese M. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr Pulmonol. 2015;50(4):410-419. doi:10.1002/ppul.23133"
},
{
"DOI": "10.1016/j.sapharm.2020.04.016",
"doi-asserted-by": "crossref",
"key": "ref28",
"unstructured": "Sarayani A, Cicali B, Henriksen CH, Brown JD. Safety signals for QT prolongation or torsades de pointes associated with azithromycin with or without chloroquine or hydroxychloroquine. Res Social Adm Pharm. 2021;17(2):483-486. doi:10.1016/j.sapharm.2020.04.016"
},
{
"DOI": "10.1177/1715163516641136",
"doi-asserted-by": "crossref",
"key": "ref29",
"unstructured": "Tisdale JE. Drug-induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management. Can Pharm J (Ott). 2016;149(3):139-152. doi:10.1177/17151 63516641136"
},
{
"DOI": "10.17826/cumj.856174",
"doi-asserted-by": "crossref",
"key": "ref30",
"unstructured": "Akkuş O, Bal T, Yaqoobi H, Bekler Ö, Akkuş G, Çabalak M. Electrocardiographic findings and cardiac safety of hydroxychloroquine +azithromycin in hospitalized patients with COVID-19. Cukurova Med J. 2021;46(2):691-698. doi:10.17826/cumj.856174"
},
{
"DOI": "10.1136/heartjnl-2020-317186",
"doi-asserted-by": "crossref",
"key": "ref31",
"unstructured": "Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106(15):1127-1131. doi:10.1136/heartjnl-2020- 317186"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "crossref",
"key": "ref32",
"unstructured": "Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585"
},
{
"DOI": "10.1016/j.eclinm.2020.100433",
"doi-asserted-by": "crossref",
"key": "ref33",
"unstructured": "Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. eClinicalMedicine. 2020;24:100433. doi:10. 1016/j.eclinm.2020.100433"
},
{
"DOI": "10.23736/S2724-5276.21.06170-X",
"doi-asserted-by": "crossref",
"key": "ref34",
"unstructured": "Hançerli Törün S, Kaba Ö, Sari Yanartas M, et al. Plasma D-dimer: a promising indicator of COVID-19 infection severity or only an acute phase reactant. Minerva Pediatr (Torino). 2021. doi:10.23736/S2724-5276.21.06170-X"
},
{
"DOI": "10.1056/NEJMc2005073",
"doi-asserted-by": "crossref",
"key": "ref35",
"unstructured": "Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663-1665. doi:10.1056/NEJMc2005073"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "http://dergipark.org.tr/en/doi/10.32322/jhsm.1625339"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Can hydroxychloroquine and azithromycin combination cause cardiac rhythm disturbances in children with COVID-19 pneumonia?",
"type": "journal-article",
"volume": "8"
}