Impact of SARS-CoV-2 infection on patients with systemic lupus erythematosus in England prior to vaccination: a retrospective observational cohort study
Adrian Paul J Rabe, Wei Jie Loke, Rubana N Kalyani, Raj Tummala, Heide A Stirnadel-Farrant, John Were, Kevin L Winthrop
BMJ Open, doi:10.1136/bmjopen-2022-071072
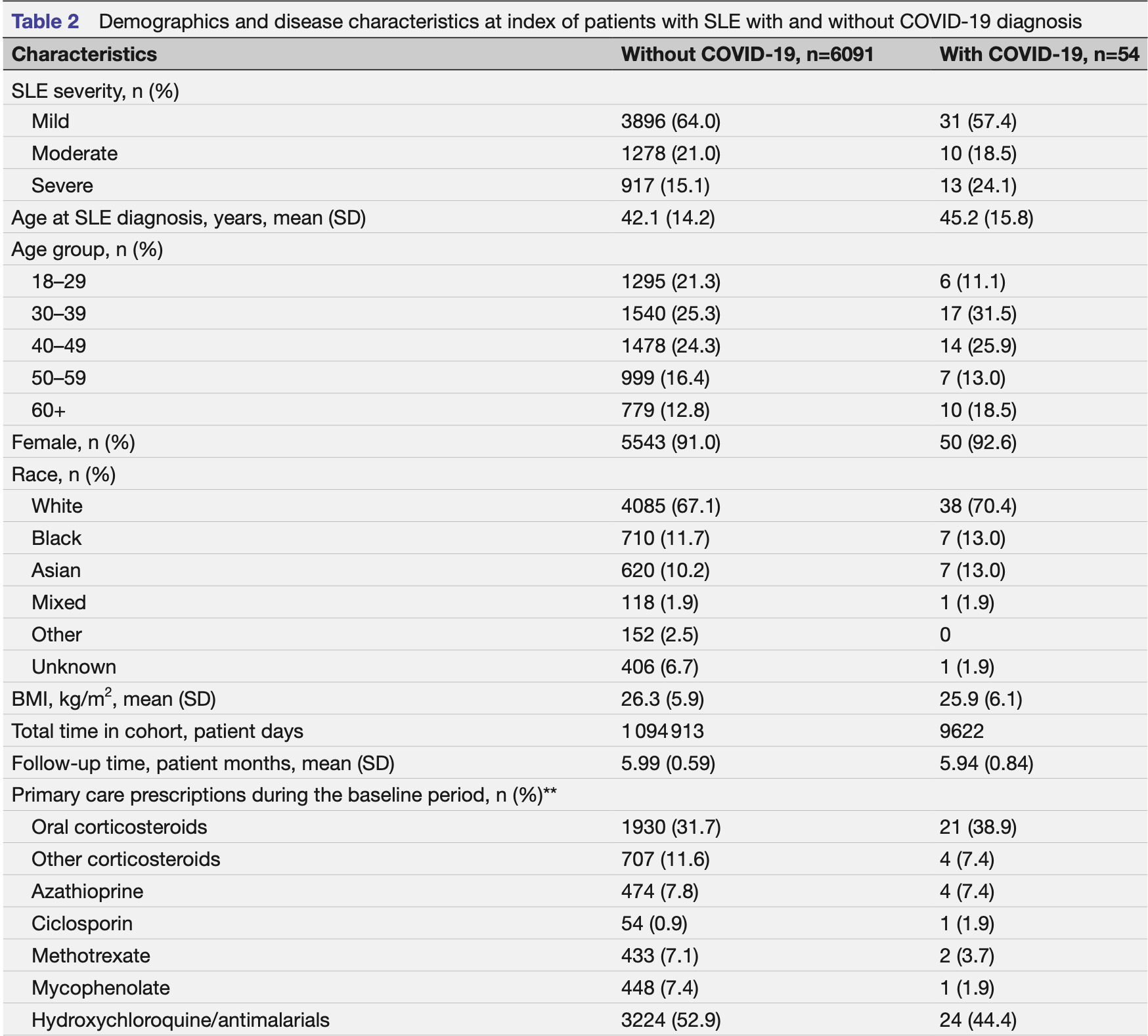
Objectives Determine the prevaccination healthcare impact of COVID-19 in patients with systemic lupus erythematosus (SLE) in England. Design Retrospective cohort study of adult patients with SLE from 1 May to 31 October 2020. Setting Clinical Practice Research Datalink (CPRD) Aurum and Hospital Episode Statistics (HES) databases from general practitioners across England combining primary care and other health-related data. Participants Overall, 6145 adults with confirmed SLE diagnosis ≥1 year prior to 1 May 2020 were included. Most patients were women (91.0%), white (67.1%), and diagnosed with SLE at age <50 (70.8%). Patients were excluded if they had a COVID-19 diagnosis before 1 May 2020. Primary and secondary outcome measures Demographics and clinical characteristics were compared. COVID-19 severity was determined by patient care required and procedure/diagnosis codes. COVID-19 cumulative incidence, hospitalisation rates, lengths of stay and mortality rates were determined and stratified by SLE and COVID-19 severity. Results Of 6145 patients, 3927 had mild, 1288 moderate and 930 severe SLE at baseline. The majority of patients with moderate to severe SLE were on oral corticosteroids and antimalarial treatments. Overall, 54/6145 (0.88%) patients with SLE acquired and were diagnosed with COVID-19, with 45 classified as mild, 6 moderate and 3 severe COVID-19. Cumulative incidence was higher in patients with severe SLE (1.4%) compared with patients classified as mild (0.8%) or moderate (0.8%). Ten COVID-19-specific hospital admissions occurred (n=6 moderate; n=4 severe). Regardless of COVID-19 status, hospital admission rates and length of stay increased with SLE severity. Of 54 patients with SLE diagnosed with COVID-19, 1 (1.9%) COVID-19-related death was recorded in a patient with both severe SLE and severe COVID-19. Conclusions SLE severity did not appear to impact COVID-19 outcomes in this study. The COVID-19 pandemic is evolving and follow-up studies are needed to understand the relationship between COVID-19 and SLE. ⇒ This study provided unique insight into the outcomes of COVID-19 for patients with systemic lupus erythematosus (SLE) before the availability of COVID-19 vaccines. ⇒ Due to the nature of a database study, there were limitations in the data captured in the system. ⇒ The number of diagnosed COVID-19 cases was low in patients with SLE. ⇒ The information about secondary care prescriptions in this population was limited.
Ethics approval This study used data that existed in an anonymised, structured format that contained no personal patient information. The study protocol was reviewed and approved by CPRD's Independent Scientific Advisory Committee (application number 21_000327) on 9 March 2021. Linkage of datasets was performed using anonymised and pseudonymised patient identification codes and was undertaken by NHS Digital, following study protocol approval. The CPRD obtains research ethics approval annually for receiving and supplying patient data for public health research from the UK's Health Research Authority Research Ethics Committee; no additional ethics approval is required for observational studies in public health research using CPRD Aurum data. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available upon reasonable request. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials. pharmacm.com/ST/Submission/Disclosure Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any..
References
Azkur, Akdis, Azkur, Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19, Allergy,
doi:10.1111/all.14364Ballow, Haga, Why do some people develop serious COVID-19 disease after infection, while others only exhibit mild symptoms?, J Allergy Clin Immunol Pract,
doi:10.1016/j.jaip.2021.01.012Bruce, 'keeffe, Farewell, Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort, Ann Rheum Dis,
doi:10.1136/annrheumdis-2013-205171Castro, Gurzenda, Macário, Characteristics, outcomes and risk factors for mortality of 522 167 patients hospitalised with COVID-19 in Brazil: a retrospective cohort study, BMJ Open,
doi:10.1136/bmjopen-2021-049089Felten, Kawka, Dubois, Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study, Lancet Rheumatol,
doi:10.1016/S2665-9913(21)00221-6Fernandez-Ruiz, Paredes, Niewold, COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease, Transl Res,
doi:10.1016/j.trsl.2020.12.007Gallagher, Dedman, Padmanabhan, The accuracy of date of death recording in the clinical practice research datalink GOLD database in England compared with the office for national statistics death registrations, Pharmacoepidemiol Drug Saf,
doi:10.1002/pds.4747Garris, Jhingran, Bass, Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan, J Med Econ,
doi:10.3111/13696998.2013.778270Gov, Uk, Coronavirus (COVID-19): guidance
Hasan, Haider, Stigler, The global case-fatality rate of COVID-19 has been declining since may 2020, Am J Trop Med Hyg,
doi:10.4269/ajtmh.20-1496Katsuyama, Tsokos, Moulton, Aberrant T cell signaling and subsets in systemic lupus erythematosus, Front Immunol,
doi:10.3389/fimmu.2018.01088Ko, Koelmeyer, Li, Predictors of infection requiring hospitalization in patients with systemic lupus erythematosus: a time-to-event analysis, Semin Arthritis Rheum,
doi:10.1016/j.semarthrit.2022.152099Langham, Barut, Samnaliev, Disease severity, flares and treatment patterns in adults with systemic lupus erythematosus in the UK: a real-world observational retrospective cohort analysis, Rheumatol Adv Pract,
doi:10.1093/rap/rkab061Liu, Davidson, Taming lupus-a new understanding of pathogenesis is leading to clinical advances, Nat Med,
doi:10.1038/nm.2752Lupus Uk, Lupus & Coronavirus (COVID-19
Martindale, Pilbeam, Mableson, Perspectives on COVID-19 testing policies and practices: a qualitative study with scientific advisors and NHS health care workers in England, BMC Public Health,
doi:10.1186/s12889-021-11285-8Mehta, Gasparyan, Zimba, Systemic lupus erythematosus in the light of the COVID-19 pandemic: infection, vaccination, and impact on disease management, Clin Rheumatol,
doi:10.1007/s10067-022-06227-7Murphy, Scott, Salisbury, Implementation of remote consulting in UK primary care following the COVID-19 pandemic: a mixed-methods longitudinal study, Br J Gen Pract,
doi:10.3399/BJGP.2020.0948Nhs, Healthcare associated COVID-19 infections -further action
Nhs, NHS offers COVID jab to clinically vulnerable and people 65 to 69
Nhs, Shielded patient list
Nune, Iyengar, Ahmed, Impact of COVID-19 on rheumatology practice in the UK-a pan-regional rheumatology survey, Clin Rheumatol,
doi:10.1007/s10067-021-05601-1Park, Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19), Clin Exp Pediatr,
doi:10.3345/cep.2020.00493Sawah, Zhang, Zhu, Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort, Lupus Sci Med,
doi:10.1136/lupus-2014-000066Saxena, Engel, Banbury, Breakthrough SARS-CoV-2 infections, morbidity, and seroreactivity following initial COVID-19 vaccination series and additional dose in patients with SLE in New York City, Lancet Rheumatol,
doi:10.1016/S2665-9913(22)00190-4Sheane, Gladman, Su, Disease outcomes in glucocorticosteroid-naive patients with systemic lupus erythematosus, Arthritis Care Res,
doi:10.1002/acr.22938Speyer, Li, Guan, Comparison of an administrative algorithm for SLE disease severity to clinical SLE disease activity index scores, Rheumatol Int,
doi:10.1007/s00296-019-04477-4Toyoshima, Nemoto, Matsumoto, SARS-CoV-2 genomic variations associated with mortality rate of COVID-19, J Hum Genet,
doi:10.1038/s10038-020-0808-9Tsang, Chan, Cho, An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies, Expert Rev Anti Infect Ther,
doi:10.1080/14787210.2021.1863146Uk, Diagnosis, None
Wolf, Dedman, Campbell, Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum, Int J Epidemiol,
doi:10.1093/ije/dyz034DOI record:
{
"DOI": "10.1136/bmjopen-2022-071072",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2022-071072",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>Determine the prevaccination healthcare impact of COVID-19 in patients with systemic lupus erythematosus (SLE) in England.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Retrospective cohort study of adult patients with SLE from 1 May to 31 October 2020.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Clinical Practice Research Datalink (CPRD) Aurum and Hospital Episode Statistics (HES) databases from general practitioners across England combining primary care and other health-related data.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Overall, 6145 adults with confirmed SLE diagnosis ≥1 year prior to 1 May 2020 were included. Most patients were women (91.0%), white (67.1%), and diagnosed with SLE at age <50 (70.8%). Patients were excluded if they had a COVID-19 diagnosis before 1 May 2020.</jats:p></jats:sec><jats:sec><jats:title>Primary and secondary outcome measures</jats:title><jats:p>Demographics and clinical characteristics were compared. COVID-19 severity was determined by patient care required and procedure/diagnosis codes. COVID-19 cumulative incidence, hospitalisation rates, lengths of stay and mortality rates were determined and stratified by SLE and COVID-19 severity.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of 6145 patients, 3927 had mild, 1288 moderate and 930 severe SLE at baseline. The majority of patients with moderate to severe SLE were on oral corticosteroids and antimalarial treatments. Overall, 54/6145 (0.88%) patients with SLE acquired and were diagnosed with COVID-19, with 45 classified as mild, 6 moderate and 3 severe COVID-19. Cumulative incidence was higher in patients with severe SLE (1.4%) compared with patients classified as mild (0.8%) or moderate (0.8%). Ten COVID-19-specific hospital admissions occurred (n=6 moderate; n=4 severe). Regardless of COVID-19 status, hospital admission rates and length of stay increased with SLE severity. Of 54 patients with SLE diagnosed with COVID-19, 1 (1.9%) COVID-19-related death was recorded in a patient with both severe SLE and severe COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>SLE severity did not appear to impact COVID-19 outcomes in this study. The COVID-19 pandemic is evolving and follow-up studies are needed to understand the relationship between COVID-19 and SLE.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2022-071072"
],
"author": [
{
"affiliation": [],
"family": "Rabe",
"given": "Adrian Paul J",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2266-5938",
"affiliation": [],
"authenticated-orcid": false,
"family": "Loke",
"given": "Wei Jie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalyani",
"given": "Rubana N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tummala",
"given": "Raj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stirnadel-Farrant",
"given": "Heide A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Were",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Winthrop",
"given": "Kevin L",
"sequence": "additional"
}
],
"container-title": "BMJ Open",
"container-title-short": "BMJ Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T17:36:13Z",
"timestamp": 1700674573000
},
"deposited": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T17:36:30Z",
"timestamp": 1700674590000
},
"funder": [
{
"DOI": "10.13039/100004325",
"award": [
"N/A"
],
"doi-asserted-by": "publisher",
"name": "AstraZeneca"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T00:32:35Z",
"timestamp": 1700699555895
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2023,
11,
22
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 21,
"start": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T00:00:00Z",
"timestamp": 1700611200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2022-071072",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e071072",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
22
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2023112209351676000_13.11.e071072.1",
"unstructured": "World Health Organization . WHO Coronavirus (COVID-19) Dashboard 2022. Available: https://covid19.who.int/ [Accessed 9 Dec 2022]."
},
{
"DOI": "10.3345/cep.2020.00493",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.2"
},
{
"DOI": "10.4269/ajtmh.20-1496",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.3"
},
{
"DOI": "10.1038/s10038-020-0808-9",
"article-title": "SARS-CoV-2 genomic variations associated with mortality rate of COVID-19",
"author": "Toyoshima",
"doi-asserted-by": "crossref",
"first-page": "1075",
"journal-title": "J Hum Genet",
"key": "2023112209351676000_13.11.e071072.4",
"volume": "65",
"year": "2020"
},
{
"DOI": "10.1016/j.jaip.2021.01.012",
"article-title": "Why do some people develop serious COVID-19 disease after infection, while others only exhibit mild symptoms?",
"author": "Ballow",
"doi-asserted-by": "crossref",
"first-page": "1442",
"journal-title": "J Allergy Clin Immunol Pract",
"key": "2023112209351676000_13.11.e071072.5",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2021-049089",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.6",
"unstructured": "Castro MC , Gurzenda S , Macário EM , et al . Characteristics, outcomes and risk factors for mortality of 522 167 patients hospitalised with COVID-19 in Brazil: a retrospective cohort study. BMJ Open 2021;11:e049089. doi:10.1136/bmjopen-2021-049089"
},
{
"DOI": "10.1080/14787210.2021.1863146",
"article-title": "An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies",
"author": "Tsang",
"doi-asserted-by": "crossref",
"first-page": "877",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "2023112209351676000_13.11.e071072.7",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.07.012",
"article-title": "The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1284",
"journal-title": "Cell",
"key": "2023112209351676000_13.11.e071072.8",
"volume": "182",
"year": "2020"
},
{
"key": "2023112209351676000_13.11.e071072.9",
"unstructured": "Centers for Disease Control and Prevention . Symptoms of COVID-19 2022. Available: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html#print [Accessed 9 Dec 2022]."
},
{
"DOI": "10.1111/all.14364",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.10"
},
{
"DOI": "10.1016/j.cpcardiol.2020.100618",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.11"
},
{
"DOI": "10.1136/bmj.m1198",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.12",
"unstructured": "Jordan RE , Adab P , Cheng KK . Covid-19: risk factors for severe disease and death. BMJ 2020;368:m1198. doi:10.1136/bmj.m1198"
},
{
"DOI": "10.1136/annrheumdis-2019-215089",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.13"
},
{
"DOI": "10.3389/fimmu.2018.01088",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.14",
"unstructured": "Katsuyama T , Tsokos GC , Moulton VR . Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol 2018;9:1088. doi:10.3389/fimmu.2018.01088"
},
{
"DOI": "10.1016/j.it.2017.02.001",
"article-title": "Expanding the B cell-centric view of systemic lupus erythematosus",
"author": "Morawski",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "Trends Immunol",
"key": "2023112209351676000_13.11.e071072.15",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.1038/nm.2752",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.16"
},
{
"DOI": "10.1002/acr.22938",
"article-title": "Disease outcomes in glucocorticosteroid-naive patients with systemic lupus erythematosus",
"author": "Sheane",
"doi-asserted-by": "crossref",
"first-page": "252",
"journal-title": "Arthritis Care Res (Hoboken)",
"key": "2023112209351676000_13.11.e071072.17",
"volume": "69",
"year": "2017"
},
{
"DOI": "10.1136/lupus-2014-000066",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.18",
"unstructured": "Al Sawah S , Zhang X , Zhu B , et al . Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med 2015;2:e000066. doi:10.1136/lupus-2014-000066"
},
{
"DOI": "10.1136/annrheumdis-2013-205171",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.19"
},
{
"key": "2023112209351676000_13.11.e071072.20",
"unstructured": "LUPUS UK . Lupus & Coronavirus (COVID-19). Available: https://www.lupusuk.org.uk/coronavirus/#amiatrisk [Accessed 17 Mar 2023]."
},
{
"DOI": "10.1016/j.semarthrit.2020.06.012",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.21"
},
{
"DOI": "10.1016/j.trsl.2020.12.007",
"article-title": "COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease",
"author": "Fernandez-Ruiz",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Transl Res",
"key": "2023112209351676000_13.11.e071072.22",
"volume": "232",
"year": "2021"
},
{
"DOI": "10.1007/s10067-022-06227-7",
"article-title": "Systemic lupus erythematosus in the light of the COVID-19 pandemic: infection, vaccination, and impact on disease management",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "2893",
"journal-title": "Clin Rheumatol",
"key": "2023112209351676000_13.11.e071072.23",
"volume": "41",
"year": "2022"
},
{
"key": "2023112209351676000_13.11.e071072.24",
"unstructured": "Department of Health & Social Care ;. Coronavirus (COVID-19) Scaling up our testing programmes. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/878121/coronavirus-covid-19-testing-strategy.pdf [Accessed 24 May 2020]."
},
{
"DOI": "10.1136/bmj-2022-070344",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.25",
"unstructured": "Majeed A , Pollock K , Hodes S , et al . Implementation of COVID-19 vaccination in the United Kingdom. BMJ 2022:e070344. doi:10.1136/bmj-2022-070344"
},
{
"DOI": "10.1093/ije/dyz034",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.26"
},
{
"key": "2023112209351676000_13.11.e071072.27",
"unstructured": "Medicines & Healthcare Products Regulatory Agency NIHR . Clinical Practice Research Datalink. 2022. Available: https://cprd.com/"
},
{
"DOI": "10.1002/pds.4747",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.28"
},
{
"DOI": "10.1136/bmjspcare-2018-001514",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.29",
"unstructured": "Harshfield A , Abel GA , Barclay S , et al . Do GPs accurately record date of death? A UK observational analysis. BMJ Support Palliat Care 2020;10:e24. doi:10.1136/bmjspcare-2018-001514"
},
{
"DOI": "10.3111/13696998.2013.778270",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.30"
},
{
"DOI": "10.1093/rap/rkab061",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.31",
"unstructured": "Langham J , Barut V , Samnaliev M , et al . Disease severity, flares and treatment patterns in adults with systemic lupus erythematosus in the UK: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract 2021;5:rkab061. doi:10.1093/rap/rkab061"
},
{
"key": "2023112209351676000_13.11.e071072.32",
"unstructured": "Office of National Statistics . England population mid-year estimate, population estimates. 2021. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates [Accessed 24 May 2022]."
},
{
"key": "2023112209351676000_13.11.e071072.33",
"unstructured": "UK Health Security Agency . Coronavirus (COVID-19) in the UK. 2022. Available: https://coronavirus.data.gov.uk/details/cases?areaType=nation&areaName=England [Accessed 10 Apr 2022]."
},
{
"key": "2023112209351676000_13.11.e071072.34",
"unstructured": "GOV.UK . Coronavirus (COVID-19): guidance. Available: https://www.gov.uk/government/collections/coronavirus-covid-19-list-of-guidance#full-publication-update-history [Accessed 22 Mar 2023]."
},
{
"DOI": "10.1186/s12889-021-11285-8",
"doi-asserted-by": "crossref",
"key": "2023112209351676000_13.11.e071072.35",
"unstructured": "Martindale A-M , Pilbeam C , Mableson H , et al . Perspectives on COVID-19 testing policies and practices: a qualitative study with scientific advisors and NHS health care workers in England. BMC Public Health 2021;21:1216. doi:10.1186/s12889-021-11285-8"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.36"
},
{
"DOI": "10.1016/j.semarthrit.2022.152099",
"article-title": "Predictors of infection requiring hospitalization in patients with systemic lupus erythematosus: a time-to-event analysis",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "152099",
"journal-title": "Semin Arthritis Rheum",
"key": "2023112209351676000_13.11.e071072.37",
"volume": "57",
"year": "2022"
},
{
"key": "2023112209351676000_13.11.e071072.38",
"unstructured": "NHS . Shielded patient list. Available: https://digital.nhs.uk/coronavirus/shielded-patient-list#:~:text=Shielded%20Patient%20List%20(SPL)%20web,Digital%20on%2030%20June%202022 [Accessed 17 Mar 2023]."
},
{
"key": "2023112209351676000_13.11.e071072.39",
"unstructured": "GOV.UK . Twice weekly rapid testing to be available to everyone in England. 2021. Available: https://www.gov.uk/government/news/twice-weekly-rapid-testing-to-be-available-to-everyone-in-england#:~:text=Everyone%20in%20England%20will%20be,April%2C%20the%20government%20has%20announced.&text=universal%20testing%20offer-,Everyone%20in%20England%20will%20be%20able%20to%20access%20free%2C%20regular,April%2C%20the%20government%20has%20announced [Accessed 6 Apr 2023]."
},
{
"key": "2023112209351676000_13.11.e071072.40",
"unstructured": "NHS . Healthcare associated COVID-19 infections – further action. June 2020. Available: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections--further-action-24-June-2020.pdf [Accessed 6 Apr 2020]."
},
{
"DOI": "10.3399/BJGP.2020.0948",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.41"
},
{
"DOI": "10.1007/s10067-021-05601-1",
"article-title": "Impact of COVID-19 on rheumatology practice in the UK-a pan-regional rheumatology survey",
"author": "Nune",
"doi-asserted-by": "crossref",
"first-page": "2499",
"journal-title": "Clin Rheumatol",
"key": "2023112209351676000_13.11.e071072.42",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2014-206334",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.43"
},
{
"DOI": "10.1016/S0140-6736(97)04248-7",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.44"
},
{
"key": "2023112209351676000_13.11.e071072.45",
"unstructured": "Medicines & Healthcare Products Regulatory Agency CPRD . CPRD Aurum. 2022. Available: https://cprd.com/cprd-aurum-may-2022-dataset [Accessed 4 Apr 2023]."
},
{
"key": "2023112209351676000_13.11.e071072.46",
"unstructured": "LUPUS UK . Diagnosis. Available: https://www.lupusuk.org.uk/diagnosis/ [Accessed 4 Apr 2023]."
},
{
"key": "2023112209351676000_13.11.e071072.47",
"unstructured": "NHS . National data opt-out. 2020. Available: https://digital.nhs.uk/data-and-information/publications/statistical/national-data-opt-out/september-2020 [Accessed 4 Apr 2023]."
},
{
"DOI": "10.1007/s00296-019-04477-4",
"article-title": "Comparison of an administrative algorithm for SLE disease severity to clinical SLE disease activity index scores",
"author": "Speyer",
"doi-asserted-by": "crossref",
"first-page": "257",
"journal-title": "Rheumatol Int",
"key": "2023112209351676000_13.11.e071072.48",
"volume": "40",
"year": "2020"
},
{
"key": "2023112209351676000_13.11.e071072.49",
"unstructured": "UK Health Security Agency . COVID-19: the green book, Chapter 14A. Coronavirus (COVID-19) vaccination information for public health professionals. 2022. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1057798/Greenbook-chapter-14a-28Feb22.pdf"
},
{
"key": "2023112209351676000_13.11.e071072.50",
"unstructured": "NHS . NHS offers COVID jab to clinically vulnerable and people 65 to 69. Available: https://www.england.nhs.uk/2021/02/nhs-offers-covid-jab-to-clinically-vulnerable-and-people-65-to-69/ [Accessed 24 May 2022]."
},
{
"key": "2023112209351676000_13.11.e071072.51",
"unstructured": "Public Health England . Impact of COVID-19 vaccines on mortality in England: December 2020 to February 2021. In: Public Health England Report. 2021. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/972592/COVID-19_vaccine_impact_on_mortality_240321.pdf"
},
{
"DOI": "10.1038/S41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.52"
},
{
"DOI": "10.3349/ymj.2021.62.11.961",
"doi-asserted-by": "publisher",
"key": "2023112209351676000_13.11.e071072.53"
},
{
"DOI": "10.1016/S2665-9913(21)00221-6",
"article-title": "Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study",
"author": "Felten",
"doi-asserted-by": "crossref",
"first-page": "e613",
"journal-title": "Lancet Rheumatol",
"key": "2023112209351676000_13.11.e071072.54",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(22)00190-4",
"article-title": "Breakthrough SARS-CoV-2 infections, morbidity, and seroreactivity following initial COVID-19 vaccination series and additional dose in patients with SLE in New York City",
"author": "Saxena",
"doi-asserted-by": "crossref",
"first-page": "e582",
"journal-title": "Lancet Rheumatol",
"key": "2023112209351676000_13.11.e071072.55",
"volume": "4",
"year": "2022"
},
{
"key": "2023112209351676000_13.11.e071072.56",
"unstructured": "Clinical Practice Research Datalink . CORYLUS UK: A retrospective observational cohort study of the impact of COVID-19 on systemic lupus erythematosus patients in England using data from linked primary and secondary care databases. 2021. Available: https://cprd.com/protocol/corylus-uk-retrospective-observational-cohort-study-impact-covid-19-systemic-lupus [Accessed 9 Dec 2022]."
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2022-071072"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Impact of SARS-CoV-2 infection on patients with systemic lupus erythematosus in England prior to vaccination: a retrospective observational cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "13"
}