Exploring trial publication and research waste in COVID-19 randomised trials of hydroxychloroquine, corticosteroids, and vitamin D: a meta-epidemiological cohort study
Lisa Fincham, Ameer Hohlfeld, Mike Clarke, Tamara Kredo, Michael Mccaul
BMC Medical Research Methodology, doi:10.1186/s12874-023-02110-4
Background The global research response to the COVID-19 pandemic was impressive, but also led to an infodemic and considerable research waste. Registered, but unpublished trials added to this noise. We aimed to determine the proportion of registered randomised trials of common COVID-19 treatments that were published and to describe the characteristics of these trials to examine the association between trial characteristics, publication status and research waste.
Methods This meta-epidemiological cohort study used a sample of randomised trials of corticosteroids, hydroxychloroquine or vitamin D as treatments for COVID-19, registered between 1 November 2019 and 31 December 2021 and available via the WHO ICTRP portal. We searched for the trials' published results up to 20 October 2022. We extracted the trial characteristics, analysing with descriptive statistics. We performed univariate logistic regression to examine the association between trials' characteristics and publication status, followed by multiple logistic regression using significant characteristics to assess the association between trial characteristics and publication status.
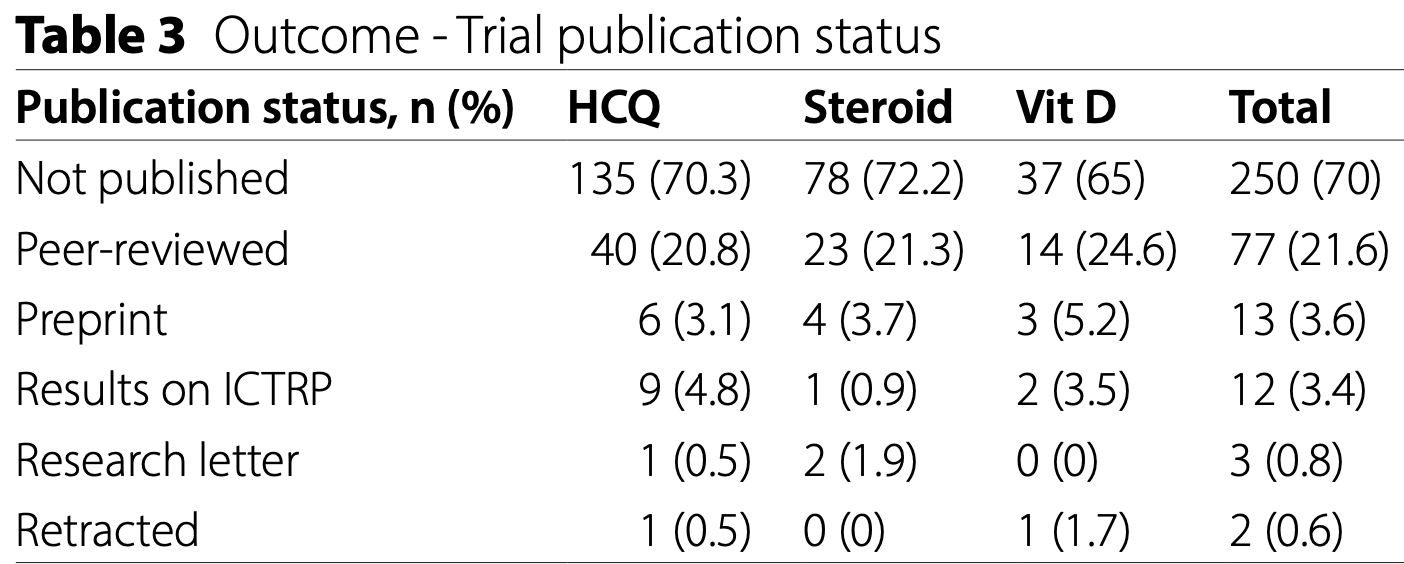
Results We identified 357 eligible trials on ICTRP. Of these, 107 (30%) had published or made their results available publicly by 20 October 2022, while 250 (70%) had not been published or shared their results publicly. Multiple logistic regression analysis showed that a larger target sample size was a significant positive predictor of publication with target sample sizes above 300 almost tripling the odds of publication (aOR: 2.75, 95% CI: 1.35 to 5.62). Conclusions Less than one third of registered trials made their results public and our findings identified that many trialists had not updated their trial registry entry with the trial status, results or both. Failure to share trial results publicly is a disservice to patients, clinicians and policy makers and adds to research waste.
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12874-023-02110-4.
Supplementary Material 1
Supplementary Material 2
Authors' contributions
Funding TK is partly supported by the Research, Evidence and Development Initiative (READ-It) project (project number 300342-104) which is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies.
Data availability The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations Ethics approval and consent to participate Not applicable (HEA-2022-26347).
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Chalmers, Fox, Increasing the incidence and influence of systematic reviews on health policy and practice, Am J Public Health
Chan, Song, Vickers, Jefferson, Dickersin et al., Increasing value and reducing waste: addressing inaccessible research, Lancet
Chen, Desai, Ross, Zhang, Chau et al., Publication and reporting of clinical trial results: Cross sectional analysis across academic medical centers, BMJ
Clarke, How can we avoid research waste during the covid-19 pandemic and plan for the future? -The BMJ
Florez, Amer, Mccaul, Lavis, Brouwers, Guidelines developed under pressure. The case of the COVID-19 low-quality "rapid" guidelines and potential solutions, J Clin Epidemiol,
doi:10.1016/j.jclinepi.2021.11.012Grainger, Bolam, Stewart, Nilsen, Evidence synthesis for tackling research waste, Nat Ecol Evol
Hopewell, Loudon, Clarke, Oxman, Dickersin, Publication bias in clinical trials due to statistical significance or direction of trial results, Cochrane Database Syst Rev
Jüni, Altman, Egger, Systematic reviews in health care: assessing the quality of controlled clinical trials, Br Med J
Krzyzanowska, Large randomized trials presented at an Oncology Meeting, JAMA
Lu, Xu, Bin, Shen, Wu et al., Characteristics and Research Waste among Randomized clinical trials in gastric Cancer, JAMA Netw Open
Lyngbakken, Berdal, Eskesen, Kvale, Olsen et al., A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics, Nat Commun [Internet,
doi:10.1038/s41467-020-19056-6Mccaul, Tovey, Young, Welch, Dewidar et al., Resources supporting trustworthy, rapid and equitable evidence synthesis and guideline development: results from the COVID-19 evidence network to support decision-making (COVID-END), J Clin Epidemiol,
doi:10.1016/j.jclinepi.2022.07.008Mitjà, Corbacho-Monné, Ubals, Tebé, Peñafiel et al., Hydroxychloroquine for early treatment of adults with mild coronavirus Disease 2019: a Randomized, Controlled Trial, Clin Infect Dis
Ross, Mulvey, Hines, Nissen, Krumholz, Trial publication after registration in ClinicalTrials.gov: a cross-sectional analysis, PLoS Med
Singh, Ryan, Kredo, Chaplin, Fletcher, Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19, Cochrane Database Syst Rev
Stroehlein, Wallqvist, Iannizzi, Mikolajewska, Metzendorf et al., Vitamin D supplementation for the treatment of COVID-19: a living systematic review, Cochrane Database Syst Rev,
doi:10.1002/14651858.CD015043Suliman, Van Den Heuvel, Suryapranata, Bisson, Seedat, Publication and non-publication of clinical trials in PTSD: an overview, Res Integr Peer Rev
Ulrich, Troxel, Carmody, Eapen, Bäcker et al., Treating COVID-19 with hydroxychloroquine (TEACH): a multicenter, double-blind randomized controlled trial in hospitalized patients, Open Forum Infect Dis
Viergever, Ghersi, The quality of registration of clinical trials, PLoS ONE
Wagner, Griesel, Mikolajewska, Mueller, Nothacker et al., Systemic corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev
Zielinski, Infodemics and infodemiology: a short history, a long future, Rev Panam Salud Publica/Pan Am J Public Heal
DOI record:
{
"DOI": "10.1186/s12874-023-02110-4",
"ISSN": [
"1471-2288"
],
"URL": "http://dx.doi.org/10.1186/s12874-023-02110-4",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The global research response to the COVID-19 pandemic was impressive, but also led to an infodemic and considerable research waste. Registered, but unpublished trials added to this noise. We aimed to determine the proportion of registered randomised trials of common COVID-19 treatments that were published and to describe the characteristics of these trials to examine the association between trial characteristics, publication status and research waste.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This meta-epidemiological cohort study used a sample of randomised trials of corticosteroids, hydroxychloroquine or vitamin D as treatments for COVID-19, registered between 1 November 2019 and 31 December 2021 and available via the WHO ICTRP portal. We searched for the trials’ published results up to 20 October 2022. We extracted the trial characteristics, analysing with descriptive statistics. We performed univariate logistic regression to examine the association between trials’ characteristics and publication status, followed by multiple logistic regression using significant characteristics to assess the association between trial characteristics and publication status.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We identified 357 eligible trials on ICTRP. Of these, 107 (30%) had published or made their results available publicly by 20 October 2022, while 250 (70%) had not been published or shared their results publicly. Multiple logistic regression analysis showed that a larger target sample size was a significant positive predictor of publication with target sample sizes above 300 almost tripling the odds of publication (aOR: 2.75, 95% CI: 1.35 to 5.62).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Less than one third of registered trials made their results public and our findings identified that many trialists had not updated their trial registry entry with the trial status, results or both. Failure to share trial results publicly is a disservice to patients, clinicians and policy makers and adds to research waste.</jats:p>\n </jats:sec>",
"alternative-id": [
"2110"
],
"article-number": "19",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 November 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "23 January 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable (HEA-2022-26347)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Fincham",
"given": "Lisa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hohlfeld",
"given": "Ameer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clarke",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kredo",
"given": "Tamara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCaul",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "BMC Medical Research Methodology",
"container-title-short": "BMC Med Res Methodol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T10:02:33Z",
"timestamp": 1706004153000
},
"deposited": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T10:04:23Z",
"timestamp": 1706004263000
},
"funder": [
{
"award": [
"300342-104"
],
"name": "Research, Evidence and Development Initiative"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
24
]
],
"date-time": "2024-01-24T00:37:39Z",
"timestamp": 1706056659809
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
23
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T00:00:00Z",
"timestamp": 1705968000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T00:00:00Z",
"timestamp": 1705968000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12874-023-02110-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12874-023-02110-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12874-023-02110-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
1,
23
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
23
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.26633/RPSP.2021.40",
"author": "C Zielinski",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Rev Panam Salud Publica/Pan Am J Public Heal",
"key": "2110_CR1",
"unstructured": "Zielinski C. Infodemics and infodemiology: a short history, a long future. Rev Panam Salud Publica/Pan Am J Public Heal. 2021;45:1–8.",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1847",
"doi-asserted-by": "publisher",
"key": "2110_CR2",
"unstructured": "Glasziou PP, Sanders S, Hoffmann T. Waste in covid-19 research. BMJ [Internet]. 2020;369(May):1–2. https://doi.org/10.1136/bmj.m1847."
},
{
"key": "2110_CR3",
"unstructured": "Mike Clarke. : How can we avoid research waste during the covid-19 pandemic and plan for the future? - The BMJ [Internet]. [cited 2022 Nov 28]. Available from: https://blogs.bmj.com/bmj/2020/04/21/mike-clarke-avoid-research-waste-covid-19-pandemic-plan-future/."
},
{
"DOI": "10.1038/s41559-020-1141-6",
"author": "MJ Grainger",
"doi-asserted-by": "publisher",
"first-page": "495",
"issue": "4",
"journal-title": "Nat Ecol Evol",
"key": "2110_CR4",
"unstructured": "Grainger MJ, Bolam FC, Stewart GB, Nilsen EB. Evidence synthesis for tackling research waste. Nat Ecol Evol. 2020;4(4):495–7.",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(09)60329-9",
"doi-asserted-by": "publisher",
"key": "2110_CR5",
"unstructured": "Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet [Internet]. 2009;374(9683):86–9. https://doi.org/10.1016/S0140-6736(09)60329-9."
},
{
"DOI": "10.2105/AJPH.2015.302915",
"author": "I Chalmers",
"doi-asserted-by": "publisher",
"first-page": "11",
"issue": "1",
"journal-title": "Am J Public Health",
"key": "2110_CR6",
"unstructured": "Chalmers I, Fox DM. Increasing the incidence and influence of systematic reviews on health policy and practice. Am J Public Health. 2016;106(1):11–3.",
"volume": "106",
"year": "2016"
},
{
"DOI": "10.1093/cid/ciaa1009",
"author": "O Mitjà",
"doi-asserted-by": "publisher",
"first-page": "E4073",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "2110_CR7",
"unstructured": "Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild coronavirus Disease 2019: a Randomized, Controlled Trial. Clin Infect Dis. 2021;73(11):E4073–81.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19056-6",
"doi-asserted-by": "publisher",
"key": "2110_CR8",
"unstructured": "Lyngbakken MN, Berdal JE, Eskesen A, Kvale D, Olsen IC, Rueegg CS et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun [Internet]. 2020;11(1):6–11. https://doi.org/10.1038/s41467-020-19056-6."
},
{
"DOI": "10.1093/ofid/ofaa446",
"doi-asserted-by": "crossref",
"key": "2110_CR9",
"unstructured": "Ulrich RJ, Troxel AB, Carmody E, Eapen J, Bäcker M, DeHovitz JA et al. Treating COVID-19 with hydroxychloroquine (TEACH): a multicenter, double-blind randomized controlled trial in hospitalized patients. Open Forum Infect Dis. 2020;7(10)."
},
{
"author": "B Singh",
"first-page": "2",
"journal-title": "Cochrane Database Syst Rev",
"key": "2110_CR10",
"unstructured": "Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2021:2.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1093/acprof:oso/9780199241323.001.0001/acprof-9780199241323-chapter-25",
"doi-asserted-by": "publisher",
"key": "2110_CR11",
"unstructured": "World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [Internet]. Reproductive Health and Human Rights Oxford University Press; Apr 17, 2003 p. 428–32. Available from: https://oxford.universitypressscholarship.com/view/https://doi.org/10.1093/acprof:oso/9780199241323.001.0001/acprof-9780199241323-chapter-25."
},
{
"DOI": "10.1001/jamanetworkopen.2021.24760",
"author": "J Lu",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "9",
"journal-title": "JAMA Netw Open",
"key": "2110_CR12",
"unstructured": "Lu J, Xu B, Bin, Shen LL, Wu D, Xue Z, Zheng HL, et al. Characteristics and Research Waste among Randomized clinical trials in gastric Cancer. JAMA Netw Open. 2021;4(9):1–14.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinepi.2022.07.008",
"doi-asserted-by": "publisher",
"key": "2110_CR13",
"unstructured": "McCaul M, Tovey D, Young T, Welch V, Dewidar O, Goetghebeur M et al. Resources supporting trustworthy, rapid and equitable evidence synthesis and guideline development: results from the COVID-19 evidence network to support decision-making (COVID-END). J Clin Epidemiol [Internet]. 2022;151:88–95. https://doi.org/10.1016/j.jclinepi.2022.07.008."
},
{
"DOI": "10.1016/j.jclinepi.2021.11.012",
"doi-asserted-by": "publisher",
"key": "2110_CR14",
"unstructured": "Florez ID, Amer YS, McCaul M, Lavis JN, Brouwers M. Guidelines developed under pressure. The case of the COVID-19 low-quality “rapid” guidelines and potential solutions. J Clin Epidemiol [Internet]. 2022;142:194–9. https://doi.org/10.1016/j.jclinepi.2021.11.012."
},
{
"DOI": "10.1016/S0140-6736(13)62296-5",
"author": "AW Chan",
"doi-asserted-by": "publisher",
"first-page": "257",
"issue": "9913",
"journal-title": "Lancet",
"key": "2110_CR15",
"unstructured": "Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257–66.",
"volume": "383",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0014701",
"author": "RF Viergever",
"doi-asserted-by": "publisher",
"first-page": "33",
"issue": "2",
"journal-title": "PLoS ONE",
"key": "2110_CR16",
"unstructured": "Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS ONE. 2011;6(2):33–6.",
"volume": "6",
"year": "2011"
},
{
"key": "2110_CR17",
"unstructured": "International Clinical Trials Registry Platform (ICTRP). [Internet]. [cited 2021 Nov 4]. Available from: https://www.who.int/clinical-trials-registry-platform."
},
{
"DOI": "10.1002/14651858.MR000006.pub3",
"doi-asserted-by": "crossref",
"key": "2110_CR18",
"unstructured": "Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009;(1)."
},
{
"author": "C Wagner",
"first-page": "8",
"journal-title": "Cochrane Database Syst Rev",
"key": "2110_CR19",
"unstructured": "Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;2021:8.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1002/14651858.CD015043",
"doi-asserted-by": "publisher",
"key": "2110_CR20",
"unstructured": "Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf M-I, Benstoem C et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev [Internet]. 2021;2021(5). https://doi.org/10.1002/14651858.CD015043."
},
{
"key": "2110_CR21",
"unstructured": "PRISMA [Internet]. [cited 2023 Oct 17]. Available from: http://www.prisma-statement.org/."
},
{
"key": "2110_CR22",
"unstructured": "ICTRP search portal -. search tips [Internet]. [cited 2022 Nov 19]. Available from: https://www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal/search-tips."
},
{
"DOI": "10.1186/s41073-019-0074-6",
"author": "S Suliman",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Res Integr Peer Rev",
"key": "2110_CR23",
"unstructured": "Suliman S, van den Heuvel L, Suryapranata A, Bisson JI, Seedat S. Publication and non-publication of clinical trials in PTSD: an overview. Res Integr Peer Rev. 2019;4(1):1–13.",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.1136/bmj.323.7303.42",
"author": "P Jüni",
"doi-asserted-by": "publisher",
"first-page": "42",
"issue": "7303",
"journal-title": "Br Med J",
"key": "2110_CR24",
"unstructured": "Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. Br Med J. 2001;323(7303):42–6.",
"volume": "323",
"year": "2001"
},
{
"DOI": "10.1001/jama.290.4.495",
"author": "MK Krzyzanowska",
"doi-asserted-by": "publisher",
"first-page": "495",
"issue": "4",
"journal-title": "JAMA",
"key": "2110_CR25",
"unstructured": "Krzyzanowska MK. Large randomized trials presented at an Oncology Meeting. JAMA. 2003;290(4):495–501.",
"volume": "290",
"year": "2003"
},
{
"DOI": "10.1371/journal.pmed.1000144",
"doi-asserted-by": "crossref",
"key": "2110_CR26",
"unstructured": "Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.gov: a cross-sectional analysis. PLoS Med. 2009;6(9)."
},
{
"author": "R Chen",
"first-page": "1",
"issue": "September 2013",
"journal-title": "BMJ",
"key": "2110_CR27",
"unstructured": "Chen R, Desai NR, Ross JS, Zhang W, Chau KH, Wayda B, et al. Publication and reporting of clinical trial results: Cross sectional analysis across academic medical centers. BMJ. 2016;352(September 2013):1–8.",
"volume": "352",
"year": "2016"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-023-02110-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health Informatics",
"Epidemiology"
],
"subtitle": [],
"title": "Exploring trial publication and research waste in COVID-19 randomised trials of hydroxychloroquine, corticosteroids, and vitamin D: a meta-epidemiological cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}