Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling
Karen Rowland Yeo, Mian Zhang, Xian Pan, Alice Ban Ke, Hannah M Jones, David Wesche, Lisa M Almond
Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955
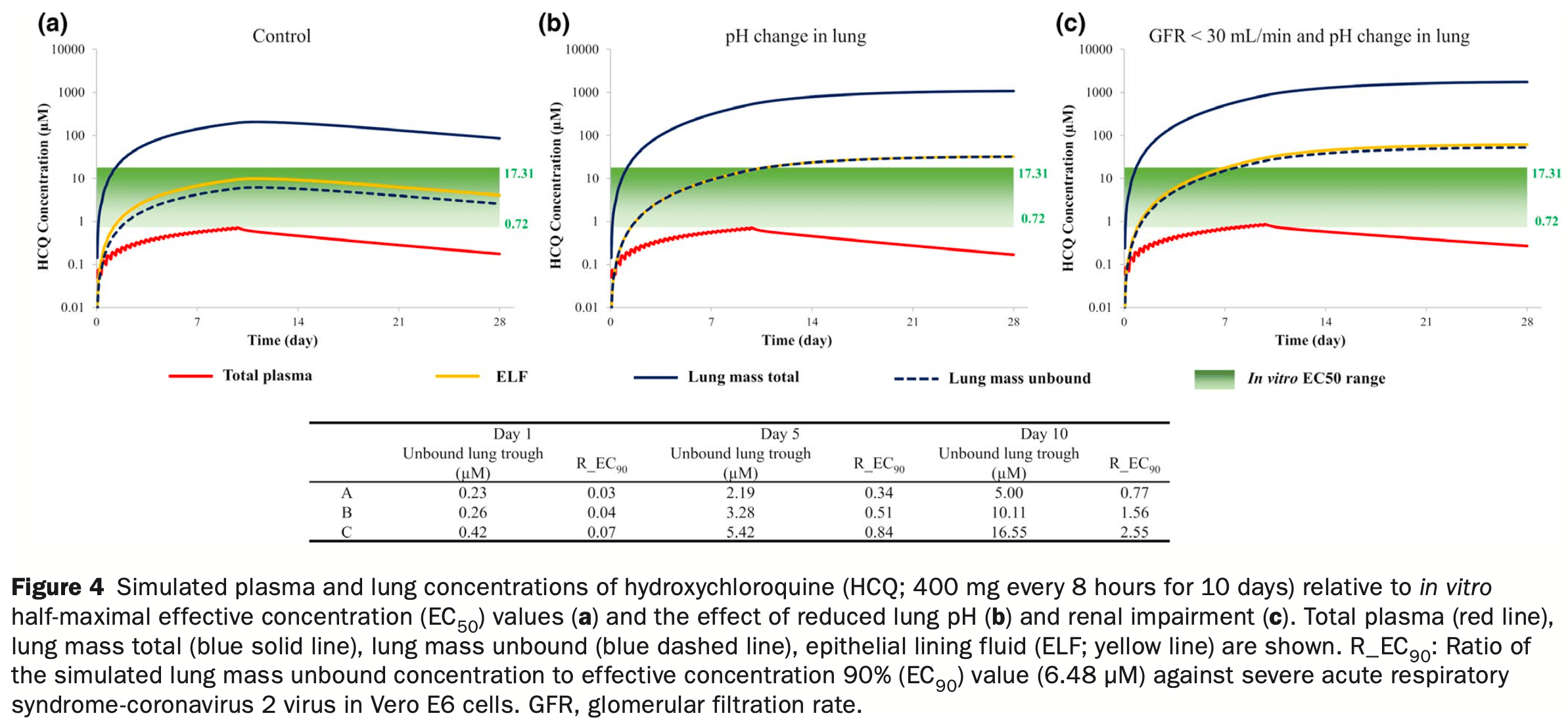
We use a mechanistic lung model to demonstrate that accumulation of chloroquine (CQ), hydroxychloroquine (HCQ), and azithromycin (AZ) in the lungs is sensitive to changes in lung pH, a parameter that can be affected in patients with coronavirus disease 2019 (COVID-19). A reduction in pH from 6.7 to 6 in the lungs, as observed in respiratory disease, led to 20-fold, 4.0-fold, and 2.7-fold increases in lung exposure of CQ, HCQ, and AZ, respectively. Simulations indicated that the relatively high concentrations of CQ and HCQ in lung tissue were sustained long after administration of the drugs had stopped. Patients with COVID-19 often present with kidney failure. Our simulations indicate that renal impairment (plus lung pH reduction) caused 30-fold, 8.0-fold, and 3.4-fold increases in lung exposures for CQ, HCQ, and AZ, respectively, with relatively small accompanying increases (20 to 30%) in systemic exposure. Although a number of different dosage regimens were assessed, the purpose of our study was not to provide recommendations for a dosing strategy, but to demonstrate the utility of a physiologically-based pharmacokinetic modeling approach to estimate lung concentrations. This, used in conjunction with robust in vitro and clinical data, can help in the assessment of COVID-19 therapeutics going forward.
Coronavirus disease 2019 (COVID-19 ) has rapidly become a global pandemic, since the outbreak was initially identified in Wuhan, China, in December 2019. The virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can infect the lower respiratory tract causing fevers, cough, and pneumonia. As new drug candidates are being investigated for treatment of COVID-19, efforts are being made to repurpose existing antimalarial drugs, as they are readily available, and have a known safety profile. Specifically, it has been reported that chloroquine (CQ) has been successful in treating SARS-CoV-2 infections in China. 1 In vitro studies have since confirmed that hydroxychloroquine (HCQ), an analog of CQ, is a more potent inhibitor of SARS-CoV-2 (5-fold to 7-fold). 2 Given that HCQ also has a more favorable safety profile than CQ during chronic dosing, a clinical study was conducted in France to determine whether HCQ (600 mg daily; 465 mg base) could be a more viable option for COVID-19
SUPPORTING INFORMATION Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
CONFLICT OF INTEREST All authors are paid employees of Certara UK Limited (Simcyp Division) or Certara Inc.
References
Adelusi, Salako, Kinetics of the distribution and elimination of chloroquine in the rat, Gen. Pharmacol
Adelusi, Salako, Tissue and blood concentrations of chloroquine following chronic administration in the rat, J. Pharm. Pharmacol
Arnold, Buckner, Hydroxychloroquine for treatment of SARS-CoV-2 infection? Improving our confidence in a modelbased approach to dose selection, Clin. Transl. Sci
Bohte, Mattie, Van Den Broek, Levels of azithromycin and alpha-1 acid glycoprotein in serum in patients with community-acquired pneumonia, Antimicrob. Agents Chemother
Cheng, Kidney impairment is associated with inhospital death of COVID-19 patients, Kidney Int
Collins, Jackson, Gustafson, Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation, J. Pharmacol. Exp. Ther
Effros, Chinard, The in vivo pH of the extravascular space of the lung, J. Clin. Invest
Fan, Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients, Clin. Infect. Dis,
doi:10.1093/cid/ciaa623.Ferrari, Cutler, Kinetics and thermodynamics of chloroquine and hydroxychloroquine transport across the human erythrocyte membrane, Biochem. Pharmacol
Fischer, Widdicombe, Mechanisms of acid and base secretion by the airway epithelium, J. Membr. Biol
Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci. Trends
Gaohua, Development of a multicompartment permeability-limited lung PBPK model and its application in predicting pulmonary pharmacokinetics of antituberculosis drugs, CPT Pharmacometrics Syst. Pharmacol
Gautret, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial, Int. J. Antimicrob. Agents,
doi:10.1016/j.ijantimicag.2020.105949.Gessner, Exhaled breath condensate acidification in acute lung injury, Respir. Med
Giudicessi, Noseworthy, Friedman, Ackerman, Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19), Mayo Clin. Proc
Hallifax, Houston, Saturable uptake of lipophilic amine drugs into isolated hepatocytes: mechanisms and consequences for quantitative clearance prediction, Drug Metab. Disposition
Jamei, A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drugdrug interaction between rosuvastatin and cyclosporine, Clin. Pharmacokinet
Johnson, Boussery, Rowland-Yeo, Tucker, Rostami-Hodjegan, A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance, Clin. Pharmacokinet
Johnson, Jamei, Rowland-Yeo, How does in vivo biliary elimination of drugs change with age? Evidence from in vitro and clinical data using a systems pharmacology approach, Drug Metab. Disposition
Jones, Rowland-Yeo, Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development, CPT Pharmacometrics Syst. Pharmacol
Katneni, Using human plasma as an assay medium in Caco-2 studies improves mass balance for lipophilic compounds, Pharm. Res
Kiem, Schentag, Interpretation of antibiotic concentration ratios measured in epithelial lining fluid, Antimicrob. Agents Chemother
Liu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Lucchi, Pharmacokinetics of azithromycin in serum, bronchial washings, alveolar macrophages and lung tissue following a single oral dose of extended or immediate release formulations of azithromycin, J. Antimicrob. Chemother
Mcchesney, Fasco, Banks, Kersch, The metabolism of chloroquine in man during and after repeated oral dosage, J. Pharmacol. Exp. Ther
Munster, Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis, Arthritis Rheum
Nožinić, Milić, Mikac, Ralić, Padovan et al., Assessment of macrolide transport using PAMPA, Caco-2 and MDCKII-hMDR1 assays, Croat. Chem. Acta
Pan, Xu, Zhang, Zhou, Wang et al., Identification of a potential mechanism of acute kidney injury during the covid-19 outbreak: a study based on single-cell transcriptome analysis, Intensive Care Med
Polak, Quantitative approach for cardiac risk assessment and interpretation in tuberculosis drug development, J. Pharmacokinet. Pharmacodyn
Rodgers, Leahy, Rowland, Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases, J. Pharm. Sci
Touret, In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication, bioRxiv,
doi:10.1101/2020.04.03.023846Viasus, Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia, J. Infect
Wan, Inflammation inhibitors were remarkably upregulated in plasma of severe acute respiratory syndrome patients at progressive phase, Proteomics
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wi, Kim, Peck, Serum albumin level as a predictor of intensive respiratory or vasopressor support in influenza A (H1N1) virus infection, Int. J. Clin. Pract
Yao, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis,
doi:10.1093/cid/ciaa237Yeo, Aarabi, Jamei, Rostami-Hodjegan, Modeling and predicting drug pharmacokinetics in patients with renal impairment, Expert Rev. Clin. Pharmacol
{ 'indexed': {'date-parts': [[2024, 7, 18]], 'date-time': '2024-07-18T05:58:29Z', 'timestamp': 1721282309826},
'reference-count': 37,
'publisher': 'Wiley',
'issue': '5',
'license': [ { 'start': { 'date-parts': [[2020, 7, 16]],
'date-time': '2020-07-16T00:00:00Z',
'timestamp': 1594857600000},
'content-version': 'vor',
'delay-in-days': 0,
'URL': 'http://creativecommons.org/licenses/by-nc/4.0/'}],
'content-domain': {'domain': ['ascpt.onlinelibrary.wiley.com'], 'crossmark-restriction': True},
'published-print': {'date-parts': [[2020, 11]]},
'abstract': '<jats:p>We use a mechanistic lung model to demonstrate that accumulation of chloroquine (CQ), '
'hydroxychloroquine (HCQ), and azithromycin (AZ) in the lungs is sensitive to changes in lung '
'pH, a parameter that can be affected in patients with coronavirus disease 2019 (COVID‐19). A '
'reduction in pH from 6.7 to 6 in the lungs, as observed in respiratory disease, led to '
'20‐fold, 4.0‐fold, and 2.7‐fold increases in lung exposure of CQ, HCQ, and AZ, respectively. '
'Simulations indicated that the relatively high concentrations of CQ and HCQ in lung tissue '
'were sustained long after administration of the drugs had stopped. Patients with COVID‐19 '
'often present with kidney failure. Our simulations indicate that renal impairment (plus lung '
'pH reduction) caused 30‐fold, 8.0‐fold, and 3.4‐fold increases in lung exposures for CQ, HCQ, '
'and AZ, respectively, with relatively small accompanying increases (20 to 30%) in systemic '
'exposure. Although a number of different dosage regimens were assessed, the purpose of our '
'study was not to provide recommendations for a dosing strategy, but to demonstrate the '
'utility of a physiologically‐based pharmacokinetic modeling approach to estimate lung '
'concentrations. This, used in conjunction with robust <jats:italic>in vitro</jats:italic> and '
'clinical data, can help in the assessment of COVID‐19 therapeutics going forward.</jats:p>',
'DOI': '10.1002/cpt.1955',
'type': 'journal-article',
'created': {'date-parts': [[2020, 6, 12]], 'date-time': '2020-06-12T22:16:08Z', 'timestamp': 1592000168000},
'page': '976-984',
'update-policy': 'http://dx.doi.org/10.1002/crossmark_policy',
'source': 'Crossref',
'is-referenced-by-count': 28,
'title': 'Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and '
'Azithromycin: Application of PBPK Modeling',
'prefix': '10.1111',
'volume': '108',
'author': [ { 'given': 'Karen',
'family': 'Rowland Yeo',
'sequence': 'first',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]},
{ 'given': 'Mian',
'family': 'Zhang',
'sequence': 'additional',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]},
{ 'given': 'Xian',
'family': 'Pan',
'sequence': 'additional',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]},
{ 'given': 'Alice',
'family': 'Ban Ke',
'sequence': 'additional',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]},
{ 'given': 'Hannah M.',
'family': 'Jones',
'sequence': 'additional',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]},
{ 'given': 'David',
'family': 'Wesche',
'sequence': 'additional',
'affiliation': [{'name': 'Certara Inc. Princeton New Jersey USA'}]},
{ 'given': 'Lisa M.',
'family': 'Almond',
'sequence': 'additional',
'affiliation': [{'name': 'Certara UK Limited (Simcyp Division) Sheffield UK'}]}],
'member': '311',
'published-online': {'date-parts': [[2020, 7, 16]]},
'reference': [ {'key': 'e_1_2_10_1_1', 'doi-asserted-by': 'publisher', 'DOI': '10.5582/bst.2020.01047'},
{ 'key': 'e_1_2_10_2_1',
'article-title': 'In vitro antiviral activity and projection of optimized dosing design '
'of hydroxychloroquine for the treatment of severe acute respiratory '
'syndrome coronavirus 2 (SARS‐CoV‐2)',
'author': 'Yao X.',
'journal-title': 'Clin. Infect. Dis.'},
{ 'key': 'e_1_2_10_3_1',
'article-title': 'Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results '
'of an open‐label non‐randomized clinical trial',
'author': 'Gautret P.',
'journal-title': 'Int. J. Antimicrob. Agents'},
{'key': 'e_1_2_10_4_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1124/dmd.107.015131'},
{'key': 'e_1_2_10_5_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1124/jpet.117.245639'},
{'key': 'e_1_2_10_6_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1016/j.kint.2020.03.005'},
{'key': 'e_1_2_10_7_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1007/s00134-020-06026-1'},
{'key': 'e_1_2_10_8_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1038/psp.2013.41'},
{'key': 'e_1_2_10_9_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1586/ecp.10.143'},
{ 'key': 'e_1_2_10_10_1',
'doi-asserted-by': 'publisher',
'DOI': '10.2165/11318160-000000000-00000'},
{'key': 'e_1_2_10_11_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1111/cts.12797'},
{'key': 'e_1_2_10_12_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1002/psp4.12034'},
{'key': 'e_1_2_10_13_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1038/s41421-019-0132-8'},
{ 'key': 'e_1_2_10_14_1',
'article-title': 'In vitro screening of a FDA approved chemical library reveals potential '
'inhibitors of SARS‐CoV‐2 replication',
'author': 'Touret F.',
'journal-title': 'bioRxiv'},
{'key': 'e_1_2_10_15_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1038/s41422-020-0282-0'},
{'key': 'e_1_2_10_16_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1124/dmd.115.068643'},
{'key': 'e_1_2_10_17_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1128/AAC.00133-06'},
{'key': 'e_1_2_10_18_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1007/s40262-013-0097-y'},
{'key': 'e_1_2_10_19_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1002/jps.20322'},
{'key': 'e_1_2_10_20_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1007/s11095-018-2493-3'},
{ 'key': 'e_1_2_10_21_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/0006-2952(91)90006-Q'},
{ 'key': 'e_1_2_10_22_1',
'first-page': '323',
'article-title': 'Assessment of macrolide transport using PAMPA, Caco‐2 and MDCKII‐hMDR1 '
'assays',
'volume': '83',
'author': 'Nožinić D.',
'year': '2010',
'journal-title': 'Croat. Chem. Acta'},
{'key': 'e_1_2_10_23_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1007/s00232-006-0861-0'},
{ 'key': 'e_1_2_10_24_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/S0954-6111(03)00225-7'},
{'key': 'e_1_2_10_25_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1172/JCI106164'},
{ 'key': 'e_1_2_10_26_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.jinf.2012.12.007'},
{'key': 'e_1_2_10_27_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1111/ijcp.12249'},
{'key': 'e_1_2_10_28_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1128/AAC.39.12.2801'},
{'key': 'e_1_2_10_29_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1002/pmic.200500638'},
{ 'key': 'e_1_2_10_30_1',
'first-page': '323',
'article-title': 'The metabolism of chloroquine in man during and after repeated oral '
'dosage',
'volume': '158',
'author': 'McChesney E.',
'year': '1967',
'journal-title': 'J. Pharmacol. Exp. Ther.'},
{'key': 'e_1_2_10_31_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1002/art.10307'},
{'key': 'e_1_2_10_32_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1093/jac/dkn032'},
{ 'key': 'e_1_2_10_33_1',
'article-title': 'Connecting hydroxychloroquine in vitro antiviral activity to in vivo '
'concentration for prediction of antiviral effect: a critical step in '
'treating COVID‐19 patients',
'author': 'Fan J.',
'journal-title': 'Clin. Infect. Dis.'},
{ 'key': 'e_1_2_10_34_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.mayocp.2020.03.024'},
{'key': 'e_1_2_10_35_1', 'doi-asserted-by': 'publisher', 'DOI': '10.1007/s10928-018-9580-2'},
{ 'key': 'e_1_2_10_36_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/0306-3623(82)90110-0'},
{ 'key': 'e_1_2_10_37_1',
'doi-asserted-by': 'publisher',
'DOI': '10.1111/j.2042-7158.1982.tb06211.x'}],
'container-title': 'Clinical Pharmacology & Therapeutics',
'original-title': [],
'language': 'en',
'link': [ { 'URL': 'https://api.wiley.com/onlinelibrary/tdm/v1/articles/10.1002%2Fcpt.1955',
'content-type': 'application/pdf',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.1955',
'content-type': 'application/pdf',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cpt.1955',
'content-type': 'application/xml',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.1955',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2023, 11, 7]],
'date-time': '2023-11-07T13:29:07Z',
'timestamp': 1699363747000},
'score': 1,
'resource': {'primary': {'URL': 'https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.1955'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2020, 7, 16]]},
'references-count': 37,
'journal-issue': {'issue': '5', 'published-print': {'date-parts': [[2020, 11]]}},
'alternative-id': ['10.1002/cpt.1955'],
'URL': 'http://dx.doi.org/10.1002/cpt.1955',
'relation': {},
'ISSN': ['0009-9236', '1532-6535'],
'subject': [],
'container-title-short': 'Clin Pharma and Therapeutics',
'published': {'date-parts': [[2020, 7, 16]]},
'assertion': [ { 'value': '2020-04-22',
'order': 0,
'name': 'received',
'label': 'Received',
'group': {'name': 'publication_history', 'label': 'Publication History'}},
{ 'value': '2020-06-06',
'order': 1,
'name': 'accepted',
'label': 'Accepted',
'group': {'name': 'publication_history', 'label': 'Publication History'}},
{ 'value': '2020-07-16',
'order': 2,
'name': 'published',
'label': 'Published',
'group': {'name': 'publication_history', 'label': 'Publication History'}}]}