The real-world effectiveness of an intranasal spray A8G6 antibody cocktail in the post-exposure prophylaxis of COVID-19
Xiaosong Li, Pai Peng, Haijun Deng, Qian Yang, Shi Chen, Benhua Li, Miao He, Zhu Yang, Ni Tang, Ailong Huang
doi:10.1101/2023.03.14.23287255
Background Due to the continuous appearance of novel SARS-CoV-2 variants that are resistant to approved antibodies and leading to the epidemic rebound, several approved neutralizing antibodies have been paused for their usage against COVID-19. Previously, we identified A8G6, an antibody combination of two synergic SARS-CoV-2 neutralizing antibodies 55A8 and 58G6, that showed broad neutralizing activities against Omicron variants. When administrated by the nasal spray delivery system, A8G6 showed promising efficacy in COVID-19 animal models and also showed favorable safety profile in preclinical models as well as in a first-in-human trial. The aim of this study is to evaluate the real-world efficacy of A8G6 neutralizing antibody nasal spray in post-exposure prevention of COVID-19.
the two groups. That is, despite participants received the A8G6 treatment, when they became infected with SARS-CoV-2, they had a comparable level of viral load compared to infected participants in the blank control group (Figure 3 ). The same analysis conducted in the per protocol set obtained the consistent results.
The effect of A8G6 on the time to the COVID-19 recovery When participants became infected with SARS-CoV-2 in both groups, RT-PCR tests or rapid antigen tests of their oropharyngeal swabs for COVID-19 and the COVID-19 related symptoms were continuously monitored and recorded. Subjects in both groups who were infected with SARS-CoV-2 during the trial period reported the conversion to COVID-19 negative by the end of the trial. The time of SARS-CoV-2 negativity between groups showed no statistical differences (p=0.946) (Figure 4 ). There is a similar result in the per protocol set.
Safety Participants receiving A8G6 treatment (n=173) were required to recorded adverse events (AEs). Thereinto, AEs reported by COVID-19 negative participants (n=161) were not correlated with COVID-19, but might be correlated with the A8G6 treatment. AEs reported by COVID-19 positive participants (n=12) might be correlated with COVID-19 or A8G6. Therefore,
Contributors AH, NT and XL designed the trial and study protocol. PP contributed to the literature search. XL and HD verified the data. PP and HD wrote the first draft manuscript. AH, NT, XL, PP, HD and ZY contributed to the data..
References
Abdul, Slenker, Monoclonal antibody therapy for high-risk coronavirus (COVID 19) patients with mild to moderate disease presentations
Abramowicz, Zucotti, Pflomm, Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the Omicron, variant
Feikin, Higdon, Abu-Raddad, 2 infection and COVID-19 disease: results of a systematic review and meta-regression
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial
Han, Wang, Li, A rapid and efficient screening system for neutralizing antibodies and its application for SARS-CoV-2
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages
Li, Han, Gu, Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants
Lin, Yue, Yang, Nasal Spray of Neutralizing Monoclonal Antibody 35B5 Confers Potential Prophylaxis Against Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern: A Small-Scale Clinical Trial
Orders, Casirivimab and imdevimab (REGEN-COV) for post-exposure prophylaxis of COVID-19
Sharif, Alzahrani, Ahmed, Skjfii, Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis
Si, Li, Safety and Effectiveness of SA58 Nasal Spray against COVID-19
Singanayagam, Hakki, Dunning, Community transmission and viral load kinetics of the SARS-CoV-2 delta (B. 1.617. 2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study
Song, Zeng, Yu, Post-Exposure Prophylaxis with SA58 (anti-COVID-19 monoclonal antibody) Nasal Spray for the prevention of symptomatic Coronavirus Disease 2019 in healthy adult workers: A randomized, single-blind, placebo-controlled clinical study
Tandon, Wu, Moore, SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: a randomized trial
Thomas, Jr, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months
Tregoning, Flight, Higham, Wang, Bfjnri, Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2
Zhang, Luo, Zhang, A cocktail containing two synergetic antibodies broadly neutralizes SARS-CoV-2 and its variants including Omicron BA, BA
Zhang, Zhang, Li, A potent neutralizing antibody provides protection against SARS-CoV-2 Omicron and Delta variants via nasal delivery
{ 'institution': [{'name': 'medRxiv'}],
'indexed': {'date-parts': [[2023, 3, 16]], 'date-time': '2023-03-16T04:40:27Z', 'timestamp': 1678941627595},
'posted': {'date-parts': [[2023, 3, 15]]},
'group-title': 'Respiratory Medicine',
'reference-count': 0,
'publisher': 'Cold Spring Harbor Laboratory',
'content-domain': {'domain': [], 'crossmark-restriction': False},
'accepted': {'date-parts': [[2023, 3, 15]]},
'abstract': '<jats:p>Background: Due to the continuous appearance of novel SARS-CoV-2 variants that are '
'resistant to approved antibodies and leading to the epidemic rebound, several approved '
'neutralizing antibodies have been paused for their usage against COVID-19. Previously, we '
'identified A8G6, an antibody combination of two synergic SARS-CoV-2 neutralizing antibodies '
'55A8 and 58G6, that showed broad neutralizing activities against Omicron variants. When '
'administrated by the nasal spray delivery system, A8G6 showed promising efficacy in COVID-19 '
'animal models and also showed favorable safety profile in preclinical models as well as in a '
'first-in-human trial. The aim of this study is to evaluate the real-world efficacy of A8G6 '
'neutralizing antibody nasal spray in post-exposure prevention of COVID-19. Methods: From '
'November 27, 2022 to January 31, 2023, an open-label, non-randomized, two-arm, '
'blank-controlled, investigator-initiated trial was conducted in Chongqing, China. High-risk '
'healthy participants (18-65 years) within 72 hours after close contact to SARS-CoV-2 infected '
'individuals were recruited and received a three-dose (1.4 mg/dose) A8G6 nasal spray treatment '
'daily or no treatment (blank control) for 7 consecutive days. The primary end points were 1) '
'the occurrence of positive SARS-CoV-2 RT-PCR cases in A8G6 treated group vs blank control '
'group at the end of day 7; 2) time to SARS-CoV-2 positive conversion at the end of day 7. The '
'secondary end points were 1) viral load of SARS-CoV-2 when participants became SARS-CoV-2 '
'positive; 2) the time from SARS-CoV-2 infection to negative COVID-19 conversion. Safety end '
'point of the nasal spray AG86 was analyzed by recording adverse events during the whole '
'course of this trial. This study was registered with Chictr.org (ChiCTR2200066416). Findings: '
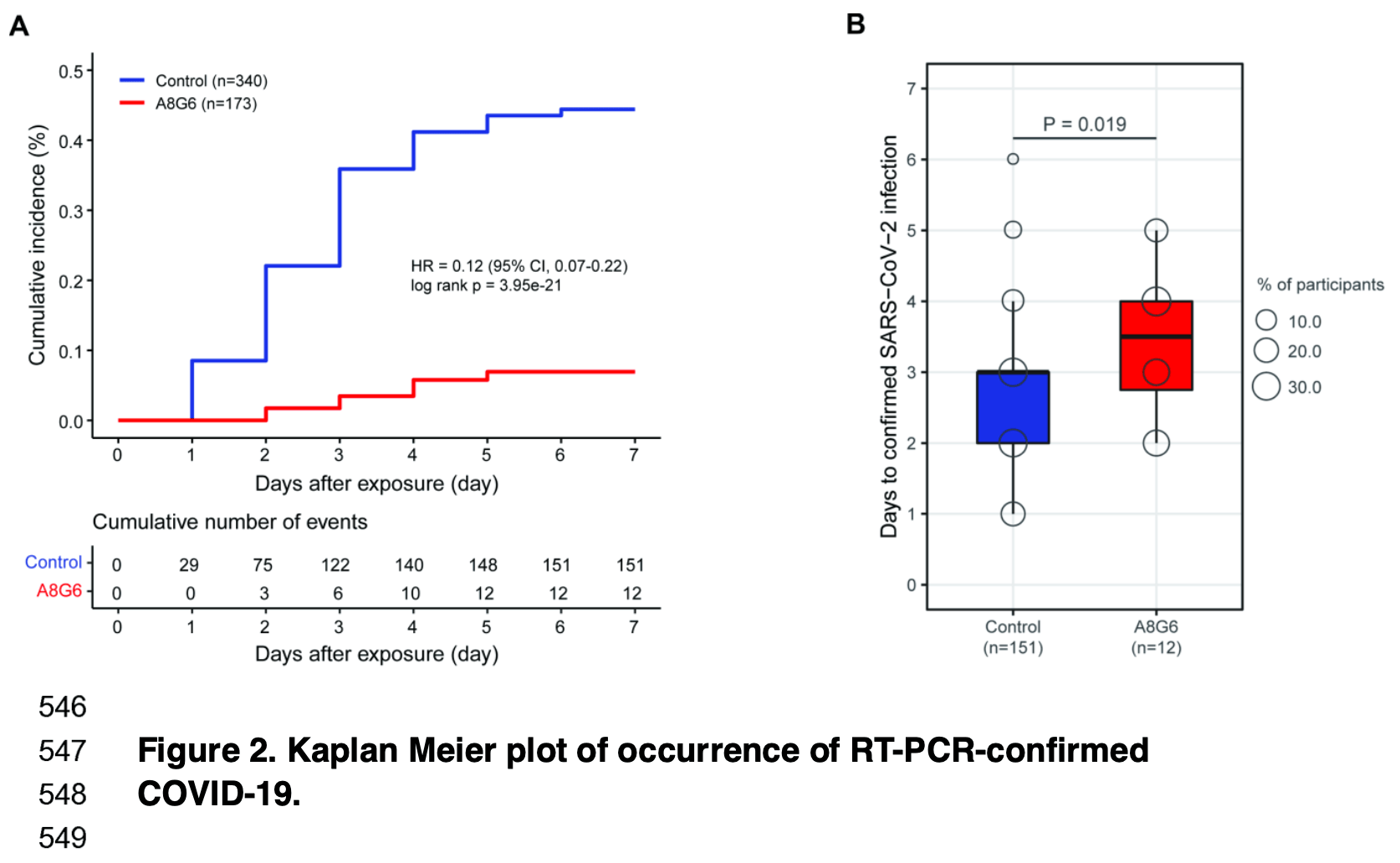
'Of 513 enrolled participants, 173 in the A8G6 treatment group and 340 in the blank-control '
'group were included in the analysis. SARS-CoV-2 infection occurred in 151/340 (44.4%) '
'subjects in the blank control group and 12/173 (6.9%) subjects with the A8G6 treatment group. '
'The result indicates that the intranasal spray A8G6 reduces the risk of SARS-CoV-2 infection '
'(HR=0.12, 95% CI, 0.07-0.22; p<0.001). The prevention efficacy of the A8G6 treatment '
'within 72-hours exposure was calculated to be 84.4% (95% CI: 74.4%-90.4%). Moreover, compared '
'to the blank-control group, the time from the SARS-CoV-2 negative to the positive COVID-19 '
'conversion was significantly longer in the AG86 treatment group (mean time: 3.4 days in the '
'A8G6 treatment group vs 2.6 days in the control group, p=0.019). In the secondary end-point '
'analysis, the A8G6 nasal treatment had no effects on the viral load at baseline SARS-CoV-2 '
'RT-PCR positivity and the time of the negative COVID-19 conversion (viral clearance). '
'Finally, 5 participants (3.1%) in the treatment group reported general adverse effects. We '
'did not observe any severe adverse effects related to the A8G6 treatment in this study. '
'Interpretation: In this study, the intranasal spray AG86 antibody cocktail showed potent '
'efficacy for prevention of SARS-CoV-2 infection in close contacts of COVID-19 '
'patients.</jats:p>',
'DOI': '10.1101/2023.03.14.23287255',
'type': 'posted-content',
'created': {'date-parts': [[2023, 3, 15]], 'date-time': '2023-03-15T20:15:17Z', 'timestamp': 1678911317000},
'source': 'Crossref',
'is-referenced-by-count': 0,
'title': 'The real-world effectiveness of an intranasal spray A8G6 antibody cocktail in the post-exposure '
'prophylaxis of COVID-19',
'prefix': '10.1101',
'author': [ {'given': 'Xiaosong', 'family': 'Li', 'sequence': 'first', 'affiliation': []},
{'given': 'Pai', 'family': 'Peng', 'sequence': 'additional', 'affiliation': []},
{'given': 'Haijun', 'family': 'Deng', 'sequence': 'additional', 'affiliation': []},
{'given': 'Qian', 'family': 'Yang', 'sequence': 'additional', 'affiliation': []},
{'given': 'Shi', 'family': 'Chen', 'sequence': 'additional', 'affiliation': []},
{'given': 'Benhua', 'family': 'Li', 'sequence': 'additional', 'affiliation': []},
{'given': 'Miao', 'family': 'He', 'sequence': 'additional', 'affiliation': []},
{'given': 'Zhu', 'family': 'Yang', 'sequence': 'additional', 'affiliation': []},
{'given': 'Ni', 'family': 'Tang', 'sequence': 'additional', 'affiliation': []},
{'given': 'Ailong', 'family': 'Huang', 'sequence': 'additional', 'affiliation': []}],
'member': '246',
'container-title': [],
'original-title': [],
'link': [ { 'URL': 'https://syndication.highwire.org/content/doi/10.1101/2023.03.14.23287255',
'content-type': 'unspecified',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2023, 3, 15]],
'date-time': '2023-03-15T20:15:18Z',
'timestamp': 1678911318000},
'score': 1,
'resource': {'primary': {'URL': 'http://medrxiv.org/lookup/doi/10.1101/2023.03.14.23287255'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2023, 3, 15]]},
'references-count': 0,
'URL': 'http://dx.doi.org/10.1101/2023.03.14.23287255',
'relation': {},
'published': {'date-parts': [[2023, 3, 15]]},
'subtype': 'preprint'}