DOI record:
{
"DOI": "10.2459/jcm.0000000000001639",
"ISSN": [
"1558-2027",
"1558-2035"
],
"URL": "http://dx.doi.org/10.2459/JCM.0000000000001639",
"author": [
{
"affiliation": [
{
"name": "Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale (EOC)"
},
{
"name": "Department of Biomedical Sciences, University of Italian Switzerland, Lugano, Switzerland"
}
],
"family": "Landi",
"given": "Antonio",
"sequence": "first"
},
{
"affiliation": [
{
"name": "IRCCS S. Maria Nascente – Fondazione Don Carlo Gnocchi ONLUS, Milan, Italy"
}
],
"family": "Morici",
"given": "Nuccia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium"
}
],
"family": "Vranckx",
"given": "Pascal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale (EOC)"
}
],
"family": "Frigoli",
"given": "Enrico",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emergency Department, ASST Great Metropolitan Hospital Niguarda"
}
],
"family": "Bonacchini",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emergency Unit, ASST Rhodense, Garbagnate Milanese"
}
],
"family": "Omazzi",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unit of General Medicine and Advanced Care, IRCCS San Raffaele Hospital, Milan, Italy"
}
],
"family": "Tresoldi",
"given": "Moreno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology, Clinica Ars Medica, Genolier Swiss Medical Network, Gravesano"
}
],
"family": "Camponovo",
"given": "Claudio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Studi Medici Riuniti & Partners, Lugano"
}
],
"family": "Moccetti",
"given": "Tiziano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inselspital, University of Bern, Bern, Switzerland"
}
],
"family": "Windecker",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale (EOC)"
},
{
"name": "Department of Biomedical Sciences, University of Italian Switzerland, Lugano, Switzerland"
},
{
"name": "Inselspital, University of Bern, Bern, Switzerland"
}
],
"family": "Valgimigli",
"given": "Marco",
"sequence": "additional"
}
],
"container-title": "Journal of Cardiovascular Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
5,
29
]
],
"date-time": "2024-05-29T11:04:55Z",
"timestamp": 1716980695000
},
"deposited": {
"date-parts": [
[
2024,
6,
3
]
],
"date-time": "2024-06-03T18:01:48Z",
"timestamp": 1717437708000
},
"indexed": {
"date-parts": [
[
2024,
6,
4
]
],
"date-time": "2024-06-04T00:19:14Z",
"timestamp": 1717460354552
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2024,
5,
28
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2024
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.2459/JCM.0000000000001639",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "565-568",
"prefix": "10.2459",
"published": {
"date-parts": [
[
2024,
5,
28
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
28
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)01585-9",
"article-title": "The Lancet Commission on lessons for the future from the COVID-19 pandemic",
"author": "Sachs",
"doi-asserted-by": "crossref",
"first-page": "1224",
"journal-title": "Lancet",
"key": "R1-20240603",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1007/s11739-020-02393-1",
"article-title": "The burden of thrombotic complications in critically ill patients with COVID-19: charting the uncharted",
"author": "Landi",
"doi-asserted-by": "crossref",
"first-page": "893",
"journal-title": "Intern Emerg Med",
"key": "R2-20240603",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2020.07.045",
"article-title": "Temporal trends in out-of-hospital cardiac arrest during the COVID-19 outbreak",
"author": "Landi",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Am J Emerg Med",
"key": "R3-20240603",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.2459/JCM.0000000000001556",
"article-title": "Edoxaban and/or colchicine in outpatients with COVID-19: rationale and design of the CONVINCE trial",
"author": "Landi",
"doi-asserted-by": "crossref",
"first-page": "920",
"journal-title": "J Cardiovasc Med (Hagerstown)",
"key": "R4-20240603",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/S2352-3026(22)00175-2",
"article-title": "Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial",
"author": "Barco",
"doi-asserted-by": "crossref",
"first-page": "e585",
"journal-title": "Lancet Haematol",
"key": "R5-20240603",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.17272",
"article-title": "Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B Randomized Clinical Trial",
"author": "Connors",
"doi-asserted-by": "crossref",
"first-page": "1703",
"journal-title": "JAMA",
"key": "R6-20240603",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif",
"doi-asserted-by": "crossref",
"first-page": "924",
"journal-title": "Lancet Respir Med",
"key": "R7-20240603",
"volume": "9",
"year": "2021"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.2459/JCM.0000000000001639"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
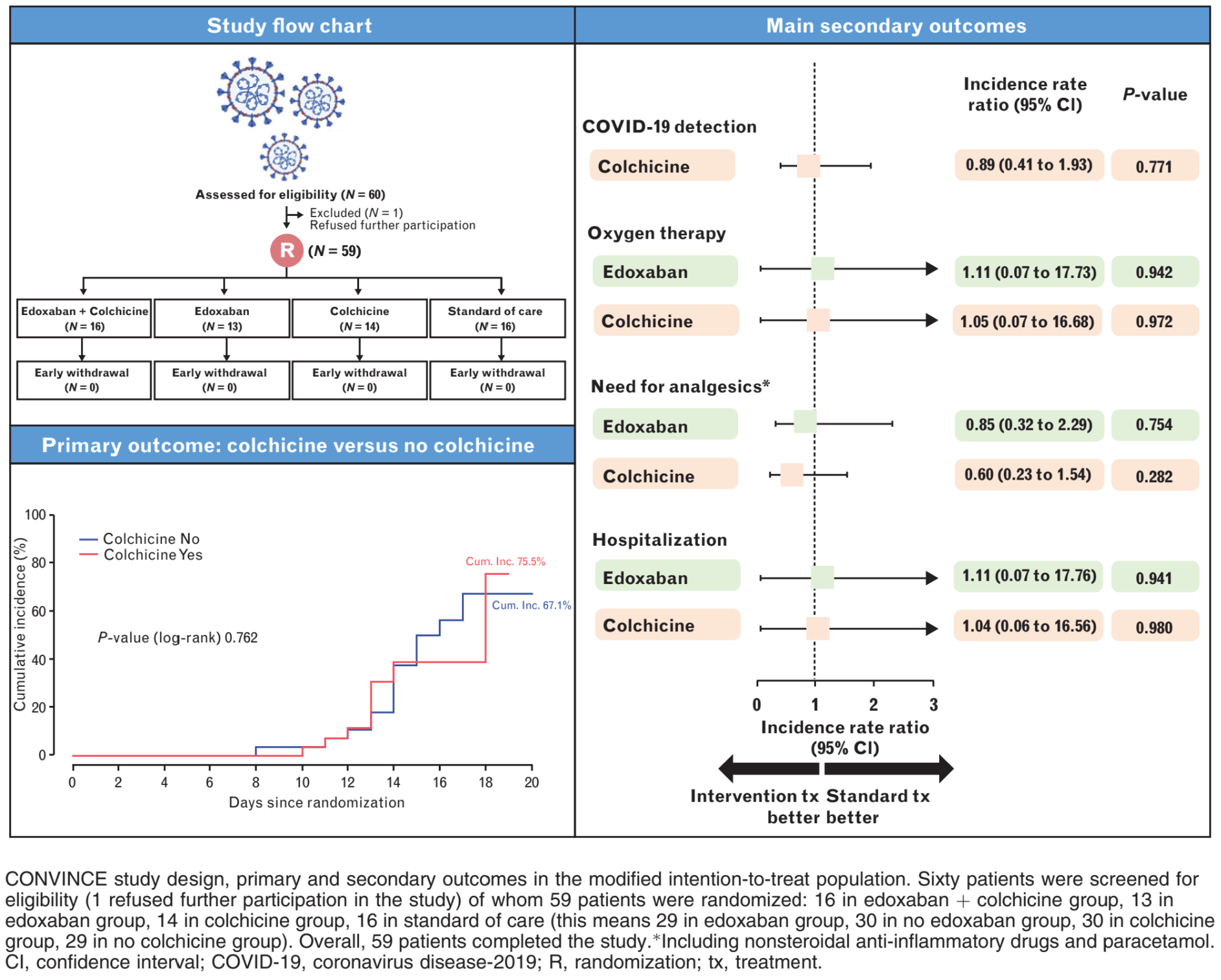
"title": "Edoxaban and/or colchicine for patients with coronavirus disease 2019 managed in the out-of-hospital setting (CONVINCE): a randomized clinical trial",
"type": "journal-article",
"volume": "25"
}