Effect of early administration of dexamethasone in patients with COVID-19 pneumonia without acute hypoxemic respiratory failure and risk of development of acute respiratory distress syndrome: EARLY-DEX COVID-19 trial
Anabel Franco-Moreno, María Soledad Acedo-Gutiérrez, Miguel Ángel Casado-Suela, Nicolás Labrador-San Martín, María De Carranza-López, Fátima Ibáñez-Estéllez, Clara Hernández-Blanco, José Jiménez-Torres, Ignacio Vallejo-Maroto, Rodolfo Romero-Pareja, Gabriela Peña-Lillo, Ismael Escobar-Rodríguez, Juan Torres-Macho
Frontiers in Medicine, doi:10.3389/fmed.2024.1385833
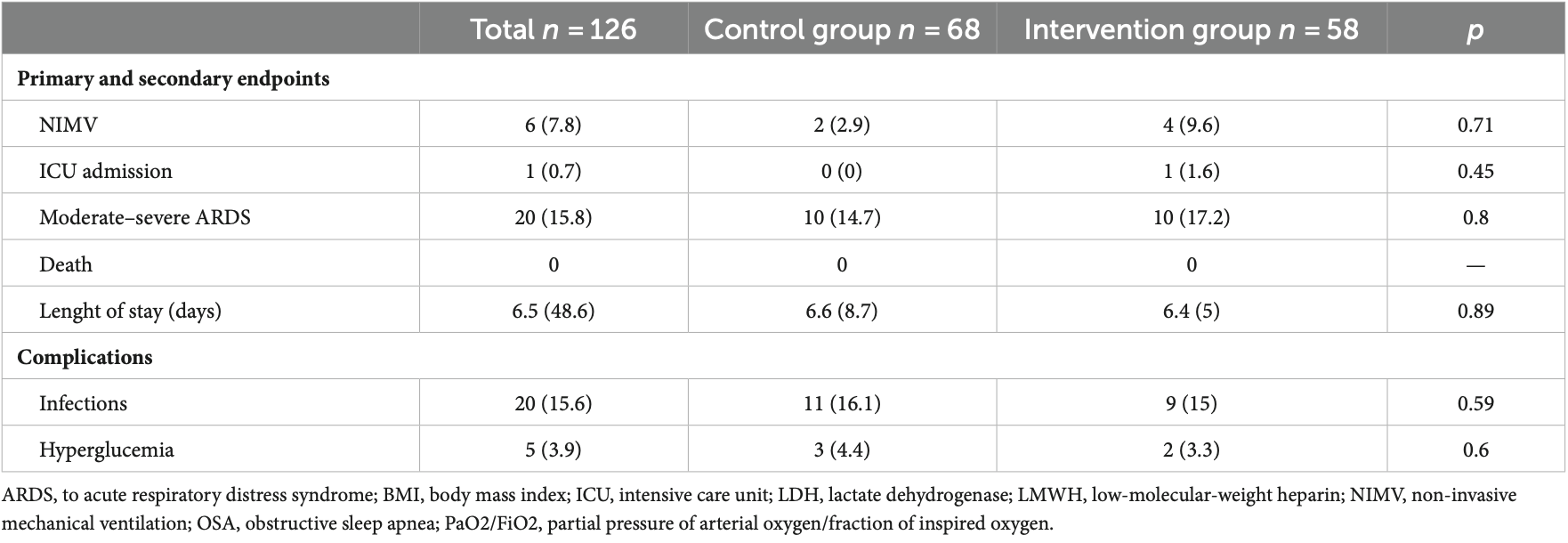
Frontiers in Medicine 02 frontiersin.org below 0.80 × 10 9 /L. Participants were randomly allocated to either receive dexamethasone or the standard care. The primary endpoints included the incidence of moderate or severe ARDS and all-cause mortality within 30 days post-enrollment. Results: One hundred twenty-six patients were randomized. Among them, 41 were female (30.8%), with a mean age of 48.8 ± 14.4 years. Ten patients in the dexamethasone group (17.2%) and ten patients in the control group (14.7%) developed moderate ARDS with no significant differences. Mechanical ventilation was required in six patients (4.7%), with four in the treatment group and two in the control group. There were no deaths during hospitalization or during follow-up. An intermediate analysis for futility showed some differences between the control and treatment groups (Z = 0.0284). However, these findings were within the margins close to the region where the null hypothesis would not be rejected.
Conclusion: In patients with COVID-19 pneumonia without oxygen needs but at risk of progressing to severe disease, early dexamethasone administration did not lead to a decrease in ARDS development.
Ethics statement The studies involving humans were approved by Clínico San Carlos University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Group members of EARLY-DEX COVID-19 research group Belén Escolano-Fernández, Nuria Alfaro-Fernández, Mateo Balado-Rico, Ana Rocío Romero-Paternina, Esther Piniella-Ruiz, Ester Alonso-Monge, and Helena Notario-Leo, Internal Medicine Department, Hospital Universitario Infanta Leonor-Virgen de la Torre, Madrid, Spain; Carlos Bibiano-Guillén and Armando Antiqueira-Pérez, Emergency Department, Hospital Universitario Infanta Leonor-Virgen de la Torre, Madrid, Spain; Noemí Cabello-Clotet, Internal Medicine Department, Hospital Universitario Clínico San Carlos, Madrid, Spain.
Author contributions
Conflict of interest Dexamethasone was provided free of charge for this study by Kern Pharma, S.L. The funding body had no input into the study design or in the writing of this manuscript.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary material..
References
Aggarwal, Mittal, Soneja, Shankar, Naik et al., Role of systemic corticosteroids in preventing hypoxia among patients with mild COVID-19: an observational study, Drug Discov Ther,
doi:10.5582/ddt.2021.01081Chalmers, Crichton, Goeminne, Cao, Humbert et al., Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline, Eur Respir J,
doi:10.1183/13993003.00048-2021Franco-Moreno, Acedo-Gutiérrez, Martín, Hernández-Blanco, Rodríguez-Olleros et al., Effect of EARLY administration of DEXamethasone in patients with COVID-19 pneumonia without acute hypoxemic respiratory failure and risk of development of acute respiratory distress syndrome (EARLY-DEX COVID-19): study protocol for a randomized controlled trial, Trials,
doi:10.1186/s13063-022-06722-xGhahramani, Tabrizi, Lankarani, Kashani, Rezaei et al., Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis, Eur J Med Res,
doi:10.1186/s40001-020-00432-3Groupjac, Murthy, Diaz, Slutsky, Villar, Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a Meta-analysis, JAMA,
doi:10.1001/jama.2020.17023Ji, Zhu, Zhong, Li, Pang et al., Association of elevated inflammatory markers and severe COVID-19: a meta-analysis, Medicine,
doi:10.1097/MD.0000000000023315Lamontagne, Stegemann, Agarwal, Agoritsas, Siemieniuk et al., A living WHO guideline on drugs to prevent covid-19, BMJ,
doi:10.1136/bmj.n526Lentner, Adams, Knutson, Zeien, Abbas et al., C-reactive protein levels associated with COVID-19 outcomes in the United States, J Osteopath Med,
doi:10.1515/jom-2021-0103Les, Loureiro-Amigo, Capdevila, Oriol, Elejalde et al., Methylprednisolone pulses in hospitalized COVID-19 patients without respiratory failure: a randomized controlled trial, Front Med,
doi:10.3389/fmed.2022.807981Martha, Wibowo, Pranata, Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis, Postgrad Med J,
doi:10.1136/postgradmedj-2020-139542Recovery Collaborative Grouphorby, Lim, Emberson, Mafham, Bell, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2021436Tang, Feng, Ni, Zhang, Liu et al., Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial, Respiration,
doi:10.1159/000512063Task Force, Ranieri, Rubenfeld, Thompson, Ferguson et al., Acute respiratory distress syndrome: the Berlin Definition, JAMA,
doi:10.1001/jama.2012.5669Tzotzos, Fischer, Fischer, Zeitlinger, Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey, Crit Care,
doi:10.1186/s13054-020-03240-7Wang, Sheng, Tu, Zhang, Association between peripheral lymphocyte count and the mortality risk of COVID-19 inpatients, BMC Pulm Med,
doi:10.1186/s12890-021-01422-9Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA,
doi:10.1001/jama.2020.2648DOI record:
{
"DOI": "10.3389/fmed.2024.1385833",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2024.1385833",
"abstract": "<jats:sec><jats:title>Introduction and objectives</jats:title><jats:p>Corticosteroids are among the drugs demonstrating a mortality benefit for coronavirus disease 2019 (COVID-19). The RECOVERY trial highlighted that dexamethasone reduced 28-day mortality for hospitalized COVID-19 patients requiring either supplemental oxygen or mechanical ventilation. It is noted that approximately 30% of COVID-19 patients, initially presenting with mild symptoms, will advance to acute respiratory distress syndrome (ARDS), especially those with detectable laboratory markers of inflammation indicative of disease progression. Our research aimed to explore the efficacy of dexamethasone in preventing the progression to ARDS in patients hospitalized with COVID-19 pneumonia who do not yet require additional oxygen but are at high risk of developing ARDS, potentially leading to a reduction in morbimortality.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In this multicenter, randomized, controlled trial, we evaluated the impact of dexamethasone on adult patients diagnosed with COVID-19 pneumonia who did not need supplementary oxygen at admission but were identified as having risk factors for ARDS. The risk of ARDS was determined based on specific criteria: elevated lactate dehydrogenase levels over 245 U/L, C-reactive protein levels exceeding 100 mg/L, and a lymphocyte count below 0.80 × 10<jats:sup>9</jats:sup>/L. Participants were randomly allocated to either receive dexamethasone or the standard care. The primary endpoints included the incidence of moderate or severe ARDS and all-cause mortality within 30 days post-enrollment.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>One hundred twenty-six patients were randomized. Among them, 41 were female (30.8%), with a mean age of 48.8 ± 14.4 years. Ten patients in the dexamethasone group (17.2%) and ten patients in the control group (14.7%) developed moderate ARDS with no significant differences. Mechanical ventilation was required in six patients (4.7%), with four in the treatment group and two in the control group. There were no deaths during hospitalization or during follow-up. An intermediate analysis for futility showed some differences between the control and treatment groups (Z = 0.0284). However, these findings were within the margins close to the region where the null hypothesis would not be rejected.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In patients with COVID-19 pneumonia without oxygen needs but at risk of progressing to severe disease, early dexamethasone administration did not lead to a decrease in ARDS development.</jats:p></jats:sec><jats:sec><jats:title>Clinical trial registration</jats:title><jats:p><jats:ext-link>ClinicalTrials.gov</jats:ext-link>, identifier NCT04836780.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2024.1385833"
],
"author": [
{
"affiliation": [],
"family": "Franco-Moreno",
"given": "Anabel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Acedo-Gutiérrez",
"given": "María Soledad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casado-Suela",
"given": "Miguel Ángel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labrador-San Martín",
"given": "Nicolás",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Carranza-López",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibáñez-Estéllez",
"given": "Fátima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández-Blanco",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiménez-Torres",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vallejo-Maroto",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero-Pareja",
"given": "Rodolfo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peña-Lillo",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Escobar-Rodríguez",
"given": "Ismael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres-Macho",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"name": "EARLY-DEX COVID-19 Research Group",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T12:37:50Z",
"timestamp": 1721306270000
},
"deposited": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T12:37:54Z",
"timestamp": 1721306274000
},
"indexed": {
"date-parts": [
[
2024,
7,
19
]
],
"date-time": "2024-07-19T00:24:31Z",
"timestamp": 1721348671372
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
7,
17
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
17
]
],
"date-time": "2024-07-17T00:00:00Z",
"timestamp": 1721174400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2024.1385833/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2024,
7,
17
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
17
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1186/s13054-020-03240-7",
"article-title": "Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey",
"author": "Tzotzos",
"doi-asserted-by": "publisher",
"first-page": "516",
"journal-title": "Crit Care",
"key": "ref1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine Storm",
"author": "Fajgenbaum",
"doi-asserted-by": "publisher",
"first-page": "2255",
"journal-title": "N Engl J Med",
"key": "ref2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"article-title": "The pathogenesis and treatment of the `cytokine Storm' in COVID-19",
"author": "Ye",
"doi-asserted-by": "publisher",
"first-page": "607",
"journal-title": "J Infect",
"key": "ref3",
"volume": "80",
"year": "2020"
},
{
"key": "ref4",
"year": ""
},
{
"key": "ref5",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n526",
"article-title": "A living WHO guideline on drugs to prevent covid-19",
"author": "Lamontagne",
"doi-asserted-by": "publisher",
"first-page": "n526",
"journal-title": "BMJ",
"key": "ref6",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "ref7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a Meta-analysis",
"author": "JAC",
"doi-asserted-by": "publisher",
"first-page": "1330",
"journal-title": "JAMA",
"key": "ref8",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s40001-020-00432-3",
"article-title": "Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis",
"author": "Ghahramani",
"doi-asserted-by": "publisher",
"first-page": "30",
"journal-title": "Eur J Med Res",
"key": "ref9",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1097/MD.0000000000023315",
"article-title": "Association of elevated inflammatory markers and severe COVID-19: a meta-analysis",
"author": "Ji",
"doi-asserted-by": "publisher",
"first-page": "e23315",
"journal-title": "Medicine (Baltimore)",
"key": "ref10",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "ref11",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1080/10408363.2020.1770685",
"article-title": "Biomarkers associated with COVID-19 disease progression",
"author": "Ponti",
"doi-asserted-by": "publisher",
"first-page": "389",
"journal-title": "Crit Rev Clin Lab Sci",
"key": "ref12",
"volume": "57",
"year": "2020"
},
{
"DOI": "10.1136/postgradmedj-2020-139542",
"article-title": "Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis",
"author": "Martha",
"doi-asserted-by": "publisher",
"first-page": "422",
"journal-title": "Postgrad Med J",
"key": "ref13",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.1515/jom-2021-0103",
"article-title": "C-reactive protein levels associated with COVID-19 outcomes in the United States",
"author": "Lentner",
"doi-asserted-by": "publisher",
"first-page": "869",
"journal-title": "J Osteopath Med",
"key": "ref14",
"volume": "121",
"year": "2021"
},
{
"DOI": "10.1186/s12890-021-01422-9",
"article-title": "Association between peripheral lymphocyte count and the mortality risk of COVID-19 inpatients",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "55",
"journal-title": "BMC Pulm Med",
"key": "ref15",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1186/s13063-022-06722-x",
"article-title": "Effect of EARLY administration of DEXamethasone in patients with COVID-19 pneumonia without acute hypoxemic respiratory failure and risk of development of acute respiratory distress syndrome (EARLY-DEX COVID-19): study protocol for a randomized controlled trial",
"author": "Franco-Moreno",
"doi-asserted-by": "publisher",
"first-page": "784",
"journal-title": "Trials",
"key": "ref16",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1001/jama.2013.281053",
"article-title": "World medical association declaration of Helsinki: ethical principles for medical research involving human subjects",
"doi-asserted-by": "publisher",
"first-page": "2191",
"journal-title": "JAMA",
"key": "ref17",
"volume": "310",
"year": "2013"
},
{
"key": "ref18",
"year": ""
},
{
"key": "ref19",
"year": ""
},
{
"DOI": "10.1001/jama.2012.5669",
"article-title": "Acute respiratory distress syndrome: the Berlin Definition",
"author": "Definition Task Force",
"doi-asserted-by": "publisher",
"first-page": "2526",
"journal-title": "JAMA",
"key": "ref20",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1159/000512063",
"article-title": "Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial",
"author": "Tang",
"doi-asserted-by": "publisher",
"first-page": "116",
"journal-title": "Respiration",
"key": "ref21",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1183/13993003.00048-2021",
"article-title": "Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline",
"author": "Chalmers",
"doi-asserted-by": "publisher",
"first-page": "2100048",
"journal-title": "Eur Respir J",
"key": "ref22",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2023.8516",
"article-title": "Dexamethasone for inpatients with COVID-19 in a National Cohort",
"author": "Mourad",
"doi-asserted-by": "publisher",
"first-page": "e238516",
"journal-title": "JAMA Netw Open",
"key": "ref23",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2022.807981",
"article-title": "Methylprednisolone pulses in hospitalized COVID-19 patients without respiratory failure: a randomized controlled trial",
"author": "Les",
"doi-asserted-by": "publisher",
"first-page": "807981",
"journal-title": "Front Med (Lausanne)",
"key": "ref24",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.5582/ddt.2021.01081",
"article-title": "Role of systemic corticosteroids in preventing hypoxia among patients with mild COVID-19: an observational study",
"author": "Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "273",
"journal-title": "Drug Discov Ther",
"key": "ref25",
"volume": "15",
"year": "2021"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2024.1385833/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of early administration of dexamethasone in patients with COVID-19 pneumonia without acute hypoxemic respiratory failure and risk of development of acute respiratory distress syndrome: EARLY-DEX COVID-19 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "11"
}