Ivermectin role in COVID-19 treatment (IRICT): single-center, adaptive, randomized, double-blind, placebo-controlled, clinical trial
Ahmed Hanei Elshafie, Hozaifa Khalil Elsawah, Mohamed Hammad, Eman Mohamed Sweed, Ahmed Salah Seif, Muhammad Mostafa Abdel Ghaffar, Feisal Mahmoud Goda, Esraa M Mosalam, Mahmoud S Abdallah
Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2022.2098113
Background: To investigate the efficacy and safety of ivermectin compared to hydroxychloroquine and placebo in hospitalized moderate to severe COVID-19 patients.
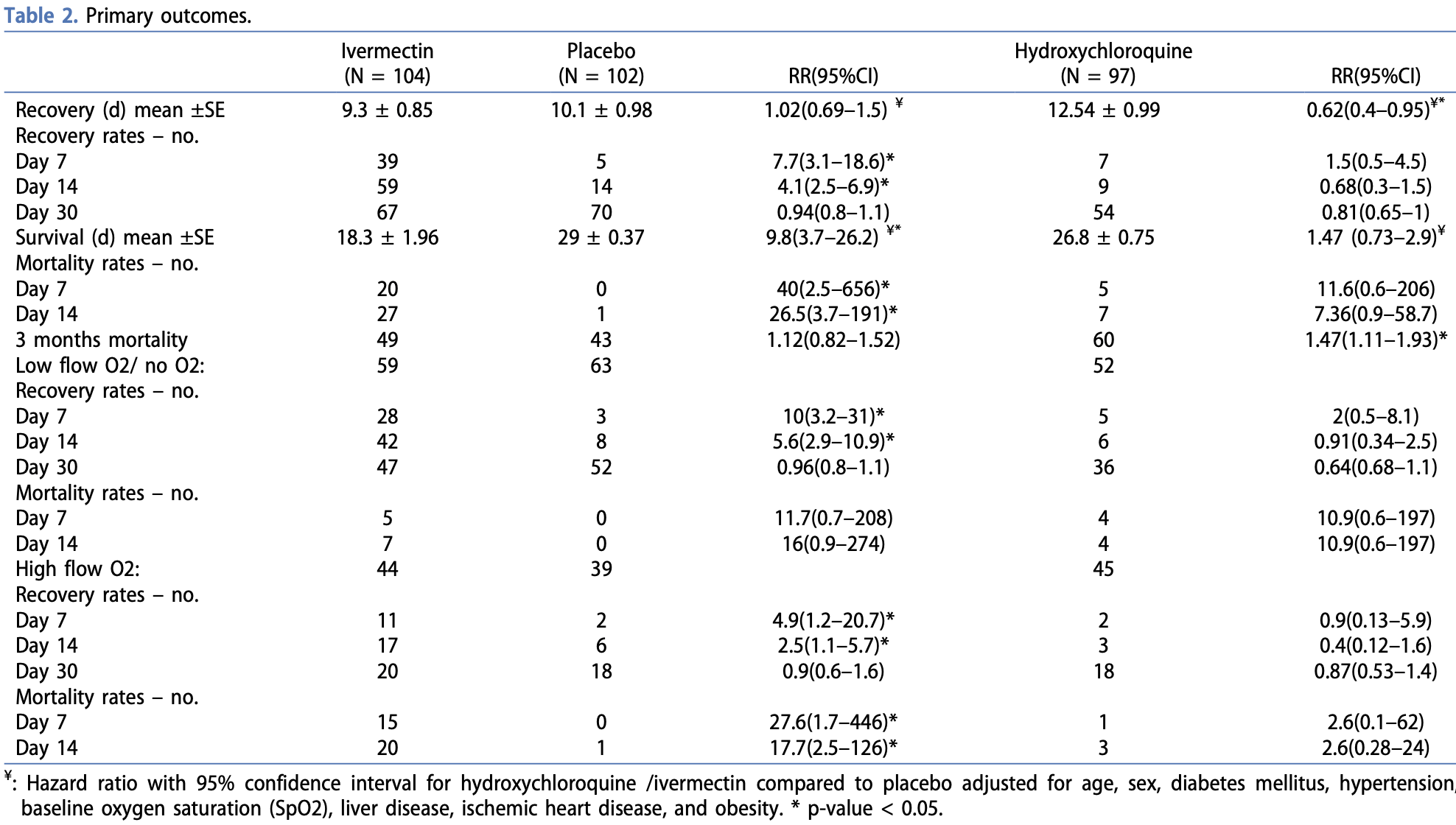
Research design and methods: The study was an adaptive, randomized, double-blinded, controlled, single-center trial. The study was a series of 3-arm comparisons between two different investigational therapeutic agents (ivermectin and hydroxychloroquine) and a placebo. There was interim monitoring to allow early stopping for futility, efficacy, or safety. Results: Ivermectin decreased survival time from 29 to 18.3 days (HR, 9.8, 95%CI, 3.7-26.2), while it did not shorten the recovery time (HR, 1.02, 95%CI, 0.69-1.5). Subgroup analysis showed an association between ivermectin-related mortality and baseline oxygen saturation level. Moreover, stratified groups showed higher risk among patients on high flow O2. Hydroxychloroquine delayed recovery from 10.1 to 12.5 days (HR, 0.62, 95%CI, 0.4-0.95) and non-significantly decreased survival time from 29 to 26.8 days (HR, 1.47, 95%CI, 0.73-2.9). However, 3 months mortality rates were increased with hydroxychloroquine (RR, 2.05, 95%CI,). Neither ivermectin nor hydroxychloroquine increased adverse events and demonstrated safety profile compared to placebo.
Conclusions: The study recommends against using either ivermectin or hydroxychloroquine for treatment of COVID-19 in hospitalized patients with any degree of severity. Clinical trial registration: www. clinicaltrials.gov identifier is: NCT04746365.
Declaration of interests The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions Ahmed Hanei Elshafie, Hozaifa Khalil Elsawah, Eman Mohamed Sweed, Muhammad Mostafa Abdel Ghaffar and Esraa M Mosalam, and Mahmoud S. Abdallah contributed to study conception, design, data and statistical analysis and interpretation, writing, and critically reviewed the manuscript; Mohamed Hammad, Ahmed Salah Seif, and Feisal Mahmoud Goda: Contributed to data collection, analysis, and interpretation, as well as drafting and substantially revising the manuscript. All authors have agreed on the journal to which the article submitted, reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage, and agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
References
Administration, Why you should not use ivermectin to treat or prevent COVID-19? 2021
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis
Altay, Mohammadi, Lam, Current status of COVID-19 therapies and drug repositioning applications, Iscience
Axfors, Schmitt, Janiaud, Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials, Nat Commun
Caly, Druce, Catton, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res
Chandler, Serious neurological adverse events after ivermectin -do they occur beyond the indication of onchocerciasis?, Am J Trop Med Hyg
Christensen, Methodology of superiority vs. equivalence trials and non-inferiority trials, J Hepatol
Coyne, Addiss, Deaths associated with ivermectin for scabies, Lancet
De Melo, Lazarini, Larrous, Anti-COVID-19 efficacy of ivermectin in the golden hamster
Duenas-Gonzalez, The pharmacokinetic rationale of ivermectin for COVID-19 therapy
Elsawah, Elsokary, Elrazzaz, Hydroxychloroquine for treatment of nonsevere COVID-19 patients: systematic review and meta-analysis of controlled clinical trials, J Med Virol
Ferri, Giuggioli, Raimondo, COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series, Clin Rheumatol
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Geary, Ivermectin 20 years on: maturation of a wonder drug, Trends Parasitol
Group, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Guy, Dipaola, Romanelli, Rapid repurposing of drugs for COVID-19, Science
Hellwig, A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin, Int J Antimicrob Agents
Hill, Abdulamir, Ahmed, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection
Hong, Gonzalez, Nahass, Impact of hydroxychloroquine on mortality in hospitalized patients with COVID-19: systematic review and meta-analysis, Pharmacy
Hossain, Rahman, Repurposing therapeutic agents against SARS-CoV-2 infection: most promising and neoteric progress, Expert Rev Anti Infect Ther
Jans, Martin, Wagstaff, Inhibitors of nuclear transport, Curr Opin Cell Biol
Kaur, Shekhar, Sharma, Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep
Kudzi, Dodoo, Mills, Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans?, BMC Med Genet
Mackenzie, Geary, Gerlach, Possible pathogenic pathways in the adverse clinical events seen following ivermectin administration to onchocerciasis patients, Filaria J
Mudatsir, Fajar, Wulandari, Predictors of COVID-19 severity: a systematic review and meta-analysis
Mudatsir, Yufika, Nainu, Antiviral activity of ivermectin against SARS-CoV-2: an old-fashioned dog with a new trick-a literature review, Sci Pharm
Murdaca, Spanò, Contatore, Pharmacogenetics of etanercept: role of TNF-α gene polymorphisms in improving its efficacy, Expert Opin Drug Metab Toxicol
Muñoz, Ballester, Antonijoan, Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers, PLoS Negl Trop Dis
Njoo, Hack, Oosting, C-reactive protein and interleukin-6 are elevated in onchocerciasis patients after ivermectin treatment, J Infect Dis
Ottesen, Campbell, Ivermectin in human medicine, J Antimicrob Chemother
Peña-Silva, Duffull, Steer, Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19
Plaquenil, None
Rainsford, Parke, Clifford-Rashotte, Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases, Inflammopharmacology
Rakedzon, Neuberger, Domb, From hydroxychloroquine to ivermectin: what are the antiviral properties of anti-parasitic drugs to combat SARS-CoV-2?, J Travel Med
Reardon, Flawed ivermectin preprint highlights challenges of COVID drug studies, Nature
Rizk, Kalantar-Zadeh, Mehra, Pharmacoimmunomodulatory therapy in COVID-19, Drugs
Rizzo, Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action, Naunyn Schmiedebergs Arch Pharmacol
Sherif, Mohamed, Ibrahim, Egyptian national guidelines for COVID −19
Soto-Becerra, Culquichicón, Hurtado-Roca, Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru
Strycharz, Yoon, Clark, A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae), J Med Entomol
Tett, Cutler, Day, Bioavailability of hydroxychloroquine tablets in healthy volunteers, Br J Clin Pharmacol
Van Eijk, Binkhorst, Bourgonje, COVID-19: immunopathology, pathophysiological mechanisms, and treatment options, J Pathol
Woldometers, COVID-19 coronavirus pandemic
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clinl Infect Dis
DOI record:
{
"DOI": "10.1080/14787210.2022.2098113",
"ISSN": [
"1478-7210",
"1744-8336"
],
"URL": "http://dx.doi.org/10.1080/14787210.2022.2098113",
"alternative-id": [
"10.1080/14787210.2022.2098113"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-11-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2022-05-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-06-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-07-04"
}
],
"author": [
{
"affiliation": [
{
"name": "Neurology and psychiatry department, Shebin Elkom teaching hospital, Menoufia university, Shebin Elkom, Menoufia, Egypt"
}
],
"family": "Elshafie",
"given": "Ahmed Hanei",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of biostatistics, high institute of public health, Alexandria university, Alexandria, Egypt"
}
],
"family": "Elsawah",
"given": "Hozaifa Khalil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Neurology and psychiatry department, Shebin Elkom teaching hospital, Menoufia university, Shebin Elkom, Menoufia, Egypt"
}
],
"family": "Hammad",
"given": "Mohamed",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3689-2648",
"affiliation": [
{
"name": "Clinical pharmacology department, Faculty of Medicine, Menoufia university, Shebin Elkom, Menoufia, Egypt"
}
],
"authenticated-orcid": false,
"family": "Sweed",
"given": "Eman Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastrohepatology and endemic medicine department, Shebin Elkom teaching hospital, Menoufia university, Shebin Elkom, Menoufia, Egypt"
}
],
"family": "Seif",
"given": "Ahmed Salah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastrohepatology and endemic medicine department, Ahmed Maher teaching hospital, Cairo, Egypt"
}
],
"family": "Abdel Ghaffar",
"given": "Muhammad Mostafa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "General surgery department, Shebin Elkom teaching hospital, Menoufia university, Shebin Elkom, Menoufia, Egypt"
}
],
"family": "Goda",
"given": "Feisal Mahmoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, Faculty of Pharmacy, Menoufia University, Shebin El-Kom, Egypt"
}
],
"family": "Mosalam",
"given": "Esraa M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3237-1792",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, University of Sadat City (USC), Sadat City, Menoufia, 32897, Egypt"
}
],
"authenticated-orcid": false,
"family": "Abdallah",
"given": "Mahmoud S.",
"sequence": "additional"
}
],
"container-title": "Expert Review of Anti-infective Therapy",
"container-title-short": "Expert Review of Anti-infective Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
5
]
],
"date-time": "2022-07-05T06:51:38Z",
"timestamp": 1657003898000
},
"deposited": {
"date-parts": [
[
2022,
7,
5
]
],
"date-time": "2022-07-05T06:51:59Z",
"timestamp": 1657003919000
},
"indexed": {
"date-parts": [
[
2022,
7,
7
]
],
"date-time": "2022-07-07T16:13:19Z",
"timestamp": 1657210399413
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
4
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/14787210.2022.2098113",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2022,
7,
4
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
4
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.isci.2020.101303",
"doi-asserted-by": "crossref",
"key": "cit0001",
"unstructured": "1.\tAltay O, Mohammadi E, Lam S, et al. Current Status of COVID-19 Therapies and Drug Repositioning Applications. Iscience. 2020 Jul;23(7)."
},
{
"key": "cit0002",
"unstructured": "2.\tWoldometers. COVID-19 CORONAVIRUS PANDEMIC 2022 [cited 2022 8 January]. Available from: https://www.worldometers.info/coronavirus/"
},
{
"DOI": "10.12688/f1000research.26186.1",
"doi-asserted-by": "crossref",
"key": "cit0003",
"unstructured": "3.\tMudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research. 2020;9:1107-1107."
},
{
"DOI": "10.1002/path.5642",
"doi-asserted-by": "crossref",
"key": "cit0004",
"unstructured": "4.\tvan Eijk LE, Binkhorst M, Bourgonje AR, et al. COVID‐19: immunopathology, pathophysiological mechanisms, and treatment options. The Journal of Pathology. 2021."
},
{
"DOI": "10.1016/j.drudis.2020.11.025",
"doi-asserted-by": "crossref",
"key": "cit0005",
"unstructured": "5.\tRizk JG, Kalantar-Zadeh K, Mehra MR, et al. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020:1-26."
},
{
"DOI": "10.1007/s10067-020-05334-7",
"doi-asserted-by": "crossref",
"key": "cit0006",
"unstructured": "6.\tFerri C, Giuggioli D, Raimondo V, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clinical Rheumatology. 2020;39(11):3195-3204."
},
{
"DOI": "10.1517/17425255.2014.970165",
"doi-asserted-by": "crossref",
"key": "cit0007",
"unstructured": "7.\tMurdaca G, Spanò F, Contatore M, et al. Pharmacogenetics of etanercept: role of TNF-α gene polymorphisms in improving its efficacy. Expert opinion on drug metabolism & toxicology. 2014;10(12):1703-1710."
},
{
"DOI": "10.1126/science.abb9332",
"doi-asserted-by": "crossref",
"key": "cit0008",
"unstructured": "8.\tGuy RK, DiPaola RS, Romanelli F, et al. Rapid repurposing of drugs for COVID-19. Science. 2020 May;368(6493):829-+."
},
{
"DOI": "10.1080/14787210.2021.1864327",
"doi-asserted-by": "crossref",
"key": "cit0009",
"unstructured": "9.\tHossain MJ, Rahman SMA. Repurposing therapeutic agents against SARS-CoV-2 infection: most promising and neoteric progress. Expert Rev Anti Infect Ther. 2021 Aug;19(8):1009-1027."
},
{
"DOI": "10.1007/s10787-015-0239-y",
"doi-asserted-by": "crossref",
"key": "cit0010",
"unstructured": "10.\tRainsford K, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231-269."
},
{
"DOI": "10.1002/jmv.26442",
"doi-asserted-by": "crossref",
"key": "cit0011",
"unstructured": "11.\tElsawah HK, Elsokary MA, Elrazzaz MG, et al. Hydroxychloroquine for treatment of nonsevere COVID‐19 patients: Systematic review and meta‐analysis of controlled clinical trials. Journal of medical virology. 2021;93(3):1265-1275."
},
{
"key": "cit0012",
"unstructured": "12.\tAxfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nature communications. 2021;12(1):1-13."
},
{
"DOI": "10.1093/jmedent/45.1.75",
"doi-asserted-by": "crossref",
"key": "cit0013",
"unstructured": "13.\tStrycharz JP, Yoon KS, Clark JM. A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae). Journal of Medical Entomology. 2008 Jan;45(1):75-81."
},
{
"DOI": "10.1016/j.pt.2005.08.014",
"doi-asserted-by": "crossref",
"key": "cit0014",
"unstructured": "14.\tGeary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends in Parasitology. 2005 Nov;21(11):530-532."
},
{
"DOI": "10.1371/journal.pntd.0006020",
"doi-asserted-by": "crossref",
"key": "cit0015",
"unstructured": "15.\tMuñoz J, Ballester MR, Antonijoan RM, et al. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl Trop Dis. 2018;12(1):e0006020-e0006020."
},
{
"DOI": "10.1007/s00210-020-01902-5",
"doi-asserted-by": "crossref",
"key": "cit0016",
"unstructured": "16.\tRizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 2020 Jul;393(7):1153-1156."
},
{
"DOI": "10.1016/j.ceb.2019.01.001",
"doi-asserted-by": "crossref",
"key": "cit0017",
"unstructured": "17.\tJans DA, Martin AJ, Wagstaff KM. Inhibitors of nuclear transport. Current Opinion in Cell Biology. 2019 Jun;58:50-60."
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "crossref",
"key": "cit0018",
"unstructured": "18.\tCaly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research. 2020 Jun;178."
},
{
"DOI": "10.3390/scipharm88030036",
"doi-asserted-by": "crossref",
"key": "cit0019",
"unstructured": "19.\tMudatsir M, Yufika A, Nainu F, et al. Antiviral Activity of Ivermectin Against SARS-CoV-2: An Old-Fashioned Dog with a New Trick—A Literature Review. Scientia Pharmaceutica. 2020;88(3)."
},
{
"DOI": "10.1007/s43440-020-00195-y",
"doi-asserted-by": "crossref",
"key": "cit0020",
"unstructured": "20.\tKaur H, Shekhar N, Sharma S, et al. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol Rep. 2021 Jan 3:1-14."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106248",
"doi-asserted-by": "crossref",
"key": "cit0021",
"unstructured": "21.\tHellwig MD, Maia A. A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin. Int J Antimicrob Agents. 2021 Jan;57(1):106248."
},
{
"DOI": "10.1101/2020.11.21.392639",
"doi-asserted-by": "crossref",
"key": "cit0022",
"unstructured": "22.\tde Melo GD, Lazarini F, Larrous F, et al. Anti-COVID-19 efficacy of ivermectin in the golden hamster. bioRxiv. 2020:2020.11.21.392639."
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"doi-asserted-by": "crossref",
"key": "cit0023",
"unstructured": "23.\tAhmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2020 Dec 2;103:214-216."
},
{
"DOI": "10.1101/2020.10.06.20208066",
"doi-asserted-by": "crossref",
"key": "cit0024",
"unstructured": "24.\tSoto-Becerra P, Culquichicón C, Hurtado-Roca Y, et al. Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru. medRxiv. 2020:2020.10.06.20208066."
},
{
"key": "cit0025",
"unstructured": "25.\tInstitutes N, Health o. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2021."
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "crossref",
"key": "cit0026",
"unstructured": "26.\tGroup RC. Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine. 2021;384(8):693-704."
},
{
"DOI": "10.1093/ofid/ofab358",
"doi-asserted-by": "crossref",
"key": "cit0027",
"unstructured": "27.\tHill A, Abdulamir A, Ahmed S, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. 2021."
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "crossref",
"key": "cit0028",
"unstructured": "28.\tGautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents. 2020;56(1):105949."
},
{
"DOI": "10.1016/j.jhep.2007.02.015",
"doi-asserted-by": "crossref",
"key": "cit0029",
"unstructured": "29.\tChristensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. Journal of hepatology. 2007;46(5):947-954."
},
{
"DOI": "10.1093/infdis/170.3.663",
"doi-asserted-by": "crossref",
"key": "cit0030",
"unstructured": "30.\tNjoo F, Hack C, Oosting J, et al. C-reactive protein and interleukin-6 are elevated in onchocerciasis patients after ivermectin treatment. Journal of Infectious Diseases. 1994;170(3):663-668."
},
{
"DOI": "10.1016/S0140-6736(05)62378-1",
"doi-asserted-by": "crossref",
"key": "cit0031",
"unstructured": "31.\tCoyne PE, Addiss DG. Deaths associated with ivermectin for scabies. The Lancet. 1997;350(9072):215-216."
},
{
"DOI": "10.1186/1475-2883-2-S1-S5",
"doi-asserted-by": "crossref",
"key": "cit0032",
"unstructured": "32.\tMackenzie CD, Geary TG, Gerlach JA. Possible pathogenic pathways in the adverse clinical events seen following ivermectin administration to onchocerciasis patients. Filaria journal. 2003;2(1):1-9."
},
{
"DOI": "10.1186/1471-2350-11-111",
"doi-asserted-by": "crossref",
"key": "cit0033",
"unstructured": "33.\tKudzi W, Dodoo AN, Mills JJ. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC medical genetics. 2010;11(1):1-8."
},
{
"DOI": "10.4269/ajtmh.17-0042",
"doi-asserted-by": "crossref",
"key": "cit0034",
"unstructured": "34.\tChandler RE. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? The American journal of tropical medicine and hygiene. 2018;98(2):382."
},
{
"key": "cit0035",
"unstructured": "35.\tSherif W, Mohamed M. E, Ibrahim H. E. Egyptian National guidelines for COVID -19 2020."
},
{
"DOI": "10.1093/jtm/taab005",
"doi-asserted-by": "crossref",
"key": "cit0036",
"unstructured": "36.\tRakedzon S, Neuberger A, Domb A, et al. From hydroxychloroquine to ivermectin: what are the anti-viral properties of anti-parasitic drugs to combat SARS-CoV-2? Journal of Travel Medicine. 2021;28(2):taab005."
},
{
"DOI": "10.3390/pharmacy8040208",
"doi-asserted-by": "crossref",
"key": "cit0037",
"unstructured": "37.\tHong TS, Gonzalez J, Nahass RG, et al. Impact of Hydroxychloroquine on Mortality in Hospitalized Patients with COVID-19: Systematic Review and Meta-Analysis. Pharmacy. 2020;8(4):208."
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "crossref",
"key": "cit0038",
"unstructured": "38.\tYao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical infectious diseases. 2020;71(15):732-739."
},
{
"DOI": "10.1111/j.1365-2125.1989.tb03439.x",
"doi-asserted-by": "crossref",
"key": "cit0039",
"unstructured": "39.\tTett S, Cutler D, Day R, et al. Bioavailability of hydroxychloroquine tablets in healthy volunteers. British journal of clinical pharmacology. 1989;27(6):771-779."
},
{
"DOI": "10.1093/jac/34.2.195",
"doi-asserted-by": "crossref",
"key": "cit0040",
"unstructured": "40.\tOttesen EA, Campbell W. Ivermectin in human medicine. Journal of antimicrobial chemotherapy. 1994;34(2):195-203."
},
{
"key": "cit0041",
"unstructured": "41.\tMerck Statement on Ivermectin use During the COVID-19 Pandemic 2021 [cited 2021 June]. Available from: https://www.merck.com/news/merck-statement-on-ivermectin-use-during-the-covid-19-pandemic/"
},
{
"key": "cit0042",
"unstructured": "42.\tPLAQUENIL: Sanofi 2017 [cited 2021 June]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf"
},
{
"DOI": "10.1038/d41586-021-02081-w",
"doi-asserted-by": "crossref",
"key": "cit0043",
"unstructured": "43.\tReardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021;596(7871):173-174."
},
{
"key": "cit0044",
"unstructured": "44.\tAdministration FDA. Why You Should Not Use Ivermectin to Treat or Prevent COVID-19? 2021 [cited 2021 12/8/2021]. Available from: https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19"
},
{
"DOI": "10.1111/bcp.14476",
"doi-asserted-by": "crossref",
"key": "cit0045",
"unstructured": "45.\tPeña‐Silva R, Duffull SB, Steer AC, et al. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID‐19. British Journal of Clinical Pharmacology. 2020."
},
{
"DOI": "10.22541/au.163513079.90720371/v1",
"doi-asserted-by": "crossref",
"key": "cit0046",
"unstructured": "46.\tDuenas-Gonzalez A. The pharmacokinetic rationale of Ivermectin for COVID-19 therapy. Authorea Preprints. 2021."
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/14787210.2022.2098113"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Ivermectin Role in COVID-19 Treatment (IRICT): single center, adaptive, randomized, double-blind, placebo controlled, clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01"
}