Virgin Coconut Oil as Adjunctive Therapy for Hospitalized COVID-19 Patients in a Tertiary Referral Hospital: A Randomized Controlled Trial
MD, MSc Marissa M Alejandria, Michelle M Leslie, PhD Dalmacio, Monica M Fresthel, MD Climacosa, Carol Stephanie, MD, MSc C Tan-Lim, MD Joseph M Abaca, Maria Llaine, MD J Callanta, Maria Elizabeth, MD, MAS P Mercado, Stephanie C Carol, MD, MSc Tan-Lim
Acta Medica Philippina, doi:10.47895/amp.vi0.7498
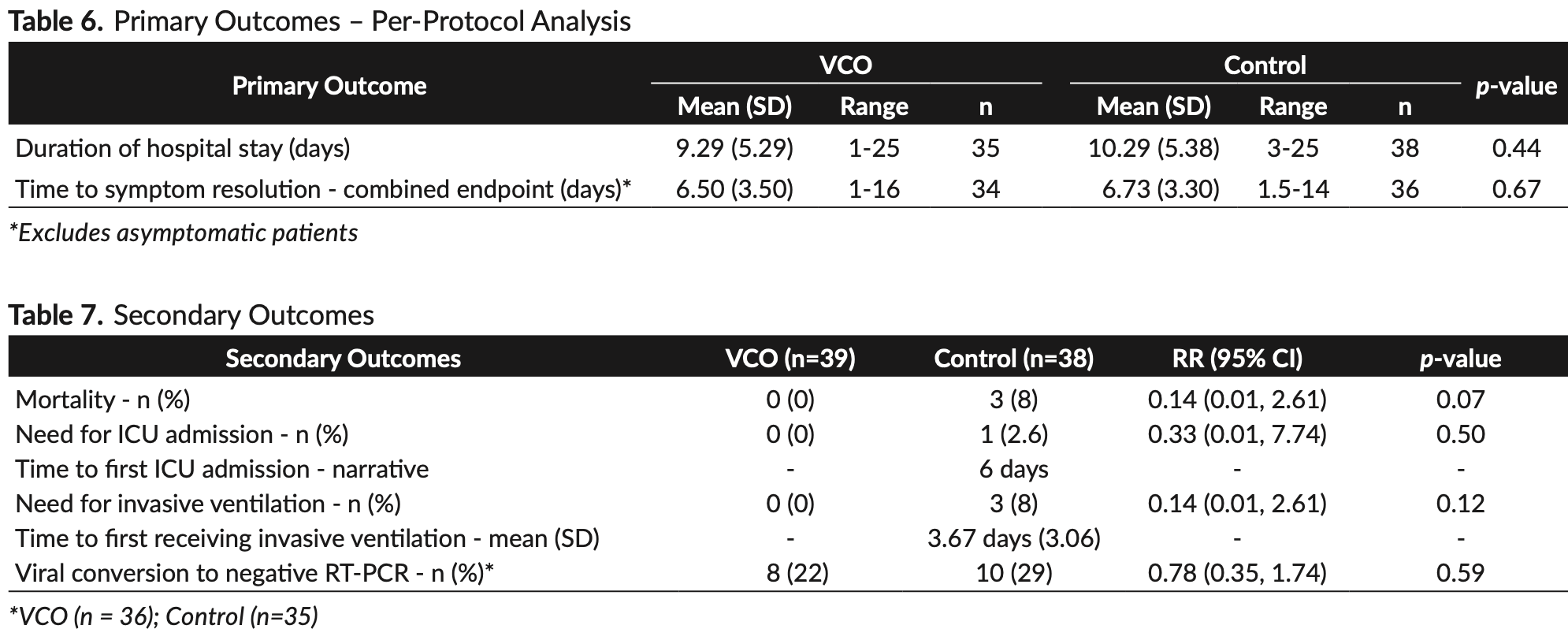
Background. Virgin coconut oil (VCO) has anti-viral and anti-inflammatory properties, making it a potential therapeutic candidate against COVID-19 infection. Objective. To determine the efficacy and safety of VCO as adjunctive therapy for hospitalized patients with COVID-19. Methods. We conducted a randomized, open-label controlled trial involving laboratory-confirmed COVID-19 patients admitted at the Philippine General Hospital. The study participants were randomized to the intervention group who received virgin coconut oil with local standard of care, or to the control group who received local standard of care alone. Results. We enrolled 39 participants into the VCO group and 38 participants into the control group. Significantly fewer participants in the VCO group had abnormal CRP levels at the end of treatment compared to control. (relative risk [RR] 0.75, 95% confidence interval [CI] 0.58 to 0.95; p=0.02) No significant difference was found in the duration of hospital stay (mean 9.33 days for VCO vs. 10.29 days for control; p=0.45) and time to symptom resolution (mean 6.8 days for VCO, vs. 6.74 days for control; p=0.91). Although the proportion of patients who developed the secondary outcomes of mortality, need for ICU admission, need for invasive ventilation, and negative viral conversion was lower in the VCO group, results did not reach statistical significance. The VCO group had larger reduction in the inflammatory markers ferritin, lactate dehydrogenase, TNF-alpha, IP-10 and IL-6, but results did not reach statistical significance. Adverse events were significantly higher in the VCO group (RR 4.87, 95% CI 1.14 to 20.79; p=0.03).
Conclusion. This clinical trial on hospitalized patients showed significant benefit in CRP levels of participants given VCO compared to control. There was no significant benefit in the use of VCO as adjunctive therapy in reducing duration of hospital stay. Larger studies are needed to conclusively demonstrate the effect of VCO on other clinical outcomes and inflammatory markers.
Statement of Authorship All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure This study was supported by the Department of Science and Technology -Philippine Council for Health Research and Development (DOST-PCHRD). The funding agency had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
Bartolotta, García, Candurra, Damonte, Effect of fatty acids on arenavirus replication: inhibition of virus production by lauric acid, Arch Virol,
doi:10.1007/s007050170146Chinwong, Chinwong, Mangklabruks, Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: a randomized crossover trial, Evid Based Complement Alternat Med,
doi:10.1155/2017/7251562Dayrit, Coconut Oil in Health and Disease: Its and Monolaurin's Potential as Cure for HIV/AIDS. XXXVII Cocotech Meeting
Famurewa, Aja, Maduagwuna, Ekeleme-Egedigwe, Ufebe et al., Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats, Biomed Pharmacother,
doi:10.1016/j.biopha.2017.12.008Hartono, Sari, Avicena, Maryani, Sukmagautama, The Effect of Curcumin and Virgin Coconut Oil Towards Cytokines Levels in COVID-19 Patients at Universitas Sebelas Maret Hospital, Surakarta, Indonesia, Pharmacogn J,
doi:10.5530/pj.2022.14.27Law, Azman, Omar, Musa, Yusoff et al., The effects of virgin coconut oil (VCO) as supplementation on quality of life (QOL) among breast cancer patients, Lipids Health Dis,
doi:10.1186/1476-511X-13-139Nacis, Capanzana, Dayrit, Tanda, Virgin coconut oil is effective in lowering C-reactive protein levels among suspect and probable cases of COVID-19, J Funct Foods,
doi:10.1016/j.jff.2021.104557Paz, Jimeno, Sy, Punzalan, Pena, The effect of virgin coconut oil on lipid profile and fasting blood sugar: a Phase I clinical trial, Philipp J Intern Med,
doi:10.3860/PJIM.V48I2.2632Tan-Lim, Martinez, Should virgin coconut oil be used in the adjunctive treatment of COVID-19?, Acta Med Philipp
Thormar, Isaacs, Brown, Barshatzky, Pessolano, Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides, Antimicrob Agents Chemother,
doi:10.1128/AAC.31.1.27Valle, Kim-Schulze, Huang, Beckmann, Nirenberg, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med,
doi:10.1038/s41591-020-1051-9Varma, Sivaprakasam, Arumugam, Dilip, Raghuraman et al., In vitro anti-inflammatory and skin protective properties of virgin coconut oil, J Tradit Complement Med,
doi:10.1016/j.jtcme.2017.06.012Vysakh, Ratheesh, Rajmohanan, Pramod, Premlal et al., Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action, Int Immunopharmacol,
doi:10.1016/j.intimp.2014.02.026Yang, Shen, Li, Yuan, Wei et al., Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19, J Allergy Clin Immunol,
doi:10.1016/j.jaci.2020.04.027DOI record:
{
"DOI": "10.47895/amp.vi0.7498",
"ISSN": [
"2094-9278"
],
"URL": "http://dx.doi.org/10.47895/amp.vi0.7498",
"container-title": "Acta Medica Philippina",
"container-title-short": "Acta Med Philipp",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T01:36:08Z",
"timestamp": 1690853768000
},
"deposited": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T01:36:08Z",
"timestamp": 1690853768000
},
"indexed": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T04:27:04Z",
"timestamp": 1690864024768
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"member": "27557",
"original-title": [],
"prefix": "10.47895",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"publisher": "University of the Philippines Manila",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://actamedicaphilippina.upm.edu.ph/index.php/acta/article/view/7498"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Virgin Coconut Oil as Adjunctive Therapy for Hospitalized COVID-19 Patients in a Tertiary Referral Hospital: A Randomized Controlled Trial",
"type": "journal-article"
}